Vitamin D Deficiency in Autism
Vitamin D is essential for neurodevelopment in the foetus and child
Vitamin D is critical for brain development and transient deficiency in the womb
results in delayed brain development
Lack
of vitamin D has been associated with reduced motivation, emotion, learning
ability and memory
Low
vitamin D is associated with delay in both gross motor skills and fine motor
skills.
Rates of vitamin D deficiency have been rapidly increasing world-wide, so too
the frequency of autism
Studies comparing vitamin D values in child found a higher incidence of autism
in children with lower vitamin D
From
2000 to 2014 in the UK there was a fifteen-fold increase in the rates of vitamin
D deficiency, paralleling the increase in the rate of autism.
Daily use of cosmetics containing SPF values of 50-60 is common.
Low vitamin D has been associated with speech delays in children
Lower vitamin D in mothers is a risk factor for having a child with autism
There is an increased rate of hypocalcemic seizures in children with low vitamin
D
Recommendations are for all pregnant and breastfeeding mothers to supplement
with vitamin D
Many mothers of children with autism report very low vitamin D levels during
pregnancy and others report extensive use of high SPF cosmetics. Many studies
have also found vitamin D deficiency to be common in ASD individuals (Bener 2017), and have suggested that low maternal vitamin D may
be a risk factor for the development of ASD, possibly via its action on fetal
brain development and altered immune status (Grant 2009). Our own studies have shown a major shift in
vitamin D processing associated SNPs (see the page on genetics). The rapid
increase in the use of sun-blocks in cosmetics, as well as the increase in SPF
values of these products are some of the few associative factors that could
account for the increase in rate of the condition. There are few other factors
that could account for such an increase, certainly not de novo mutations as has
been suggested by some (Kenney 2010).
Many studies have shown a high and increasing prevalence of vitamin D
deficiency in the general population (Diehl and Chiu, 2010) and in pregnant
mothers (Dror and Allen, 2010; Erllopta etal, 2011), and a study in the
Netherlands showed 26% of mothers to be deficient, with up to 46% of neonates
deficient (Vinkhuyzen eatl, 2016). Vitamin D deficiency is extremely prevalent
in Kuwait (54% - Al-Mutairi etal, 2012), India (Babu and Calvo, 2010), Indonesia
(45.5% of pregnant women - Ilmiawati etal, 2020), Europe (Brouwer etal, 2012);
USA (Wentz etal, 2014) and deficiency is higher in those with darker skin and
during winter (Sawicki etal, 2016). Vitamin D deficiency is also common now in
Australia and New Zealand (Shrapnel and Truswell, 2006; Quaggiotto etal, 2014).
In the US, Canada, Australia, Europe, New Zealand, and Asia, it has been
estimated that between 30 and 50 percent of children and adults suffer from
vitamin D deficiency.
Very few foods have significant levels of vitamin D, which is restricted mainly
to fatty fish, beef liver, cheese, margarines, and eggs. Potentially this
explains why vitamin D levels are signficantly lower in vegetarians than
non-vegetarians (Brooke etal, 1980).
Apart from its effect on brain development,
maternal vitamin D deficiency has also been associated with seizures in newborns
(Visser, 2005).
Whilst many are aware of the role of vitamin D in
bone health, vitamin D has a unique role in brain development, including
homeostasis, embyrogeneisis, neural differentiation, neurodevelopment, gene
regulation and immunological modulation (Duan 2013). Vitamin D also has a role
in neurotrophism, neuroprotection, and neuroplasticity (Cannell 2013), and
neuroregeneration (Gomez‐Pinedo
et al, 2020) and
vitamin D deficiency has been associated with developmental disorders and
abnormal brain development in conditions such as autism (Eyles etal, 2013;
2009).
Normal activation of vitamin D, is a well known process in which light from the
sun, or more specifically UV light from the sun shines on the skin and causes
the conversion of the precursor 7-dehydrocholesterol to be converted to vitamin
D3 - cholecalciferol. This molecule then is further processed in the liver and
converted to the inactive form 25-hydroxy-vitamin D, by the haem dependent
enzyme vitamin D-25-hydroxylase (CYP2R1).. Finally the
25-hydroxyvitamin D (Calcidiol) is activated in the kidney to form
1,25di-hydroxyvitamin D (Calcitriol).
The brain is unique amongst the other organs in that it has its own enzyme,
1a-hydroxylase, that activates 25-hydroxyvitamin-D to the active form
1,25-dihydroxy-vitamin D. The active vitamin D so produced, then binds to
specific vitamin D receptors in the brain, particularly in the hypothalamus, and
dopaminergic neurons of the substantia nigra. High levels of expression of the
1-a-hydroxylase has been in the Purkinje cells in the cerebellum (Eyles etal,
2004). Malfunctioning Purkinje cells are directly associated with the reduced
capacity for motor learning in children with autism. These cells are responsible
for fine-tuned motor control, balance, proprioception, and the vestibulo-ocular
reflex (VOR). The VOR is the reflex that stabilizes the eye movement during head
turning, such that the eyes can still focus on a target, even when the head is
turned. Many studies have found cerebellar dysfunction in people with ASD, and
post-mortem studies have shown loss of Purkinje cell volume in the majority of
autistic brains studies. Paralleling these studies is the finding by MacDonald
that children with autism are about six months behind in gross motor skills such
as running and jumping and nearly a year behind in fine motor skills such as
holding a spoon or grasping a small toy.
Mode of activation of Vitamin D in the brain, following stimulation of the eye
by 482 nm light.
Lack of vitamin D has also been associated with a loss in hippocampal volume (an
area of the brain that regulates motivation, emotion, learning and memory), and
hence low vitamin D would be associated with difficulty learning. Low vitamin D
has been associated with cognitive decline in adults (Wentz etal, 2014). Low
vitamin D in utero has been associated with autism spectrum disorder and
schizophrenia (Eyles etal, 2013; Ali etal, 2020, 2018), whilst in adults it has been associated with
depression and Alzheimer's disease. In experimental models, gestational vitamin
D deficiency has been shown to cause permanent changes in the developing brains
of rats (Levenson and Figueiroa, 2009; Feron etal, 2005), and has also been
shown to lead to persistent changes in the adult brain (Feron etal, 2005; Eyles
etal, 2012). Loading of the neonate with vitamin D occurs in utero, as very
little vitamin D is contained in milk, so an infant born from a vitamin D
deficient mother will be vitamin D deficient (Holick 2006).
Low
vitamin D has been found to impact adversely on brain development, and alters
the dopaminergic profile in the forebrain, with a reduction in COMT levels (Kesby
2009; Kesby etal, 2011). Interestingly vitamin D also promotes tyrosine
hydroxylase (TH) and tryptophan hydroxylase 2 (TPH2) expression, AND results in
a significant rise in monoamine oxidase A (MAOA) expression (Jianq 2014; Pertile
2016). This later finding is of considerable importance as MAOA is one of the
only neurotransmitter related genes that are expressed on the X-chromosome, and
hence alterations in MAO expression may provide the first reasonable hypothesis
for the increased incidence of the condition in males, who by definition only
have one X chromosome. It also supports our observations on increased
frequencies of recessive alleles in MAOA in ASD males.
Genetic studies have shown a higher rate of
the homozygous recessive genotypes in the gene coding for 1-alpha-hydoxylase in
people with autism (15.9% vs 3.8% risk ratio 4.2:1)
The importance of sun-exposure for the production of
vitamin D has been known 1822 (nearly 200 years), and particularly exposure to
UVB radiation (290-315 nm) (Holick 2006). However with the advent of
sun-protection factors in the early 1870s, and the addition of high SPF value cosmetics and the
increase in hours worked indoors, plus various sun-avoidance practices has seen a rise in the incidence of vitamin D
deficiency, and an increase in the incidence of rickets with the result that
vitamin D deficiency in children has once again reached epidemic proportions (Holick
2006). One of the potential sources of vitamin D is dairy, and so, the reduction in the consumption of dairy products,
particularly those from free range cows and the
switch to alternative products such as soy, and almond drinks, and adoption of a
vegan diet can further reduce vitamin D levels.. Vitamin D deficiency is very
common in some countries, and over 42% of Singapore residents (92),
45.5% of Saudi residents, and in 2018 over 82.5% of females in South Korea (an
increase from 76% in 2008)(93)
were found to be vitamin D deficient. Over 46% of neonates were found to be
vitamin D deficient in the Netherlands (Vinkhuysen etal, 2016). Vitamin D status decreases with increases
in weight (one of the predisposing maternal factors for giving birth to a child
with ASD). In Australia in 2012, 30% of women of child bearing age were found to
be vitamin D deficient.
Studies have shown that maternal hypovitaminosis D is increasingly associated
with a higher incidence of fetal miscarriage, preeclampsia, gestational
diabetes, bacterial vaginosis, and impaired fetal and childhood growth and
development. The development of
higher and higher SPF cosmetics, touted as being essential for the protection of
melanoma has resulted in several undesirable consequences (i). An overall
decrease in the vitamin D levels of the populations, and (ii)
A DRAMATIC and correlative increase in the rate of autsm, however (iii)
There has been an increase in the rate of melanoma from 1940 (the advent of
sunscreen above SPF10), to 2020.
The advent of high SPF value cosmetics and the
increase in hours worked indoors has seen a rise in the incidence of vitamin D
deficiency. Further, the reduction in the consumption of dairy products and the
switch to alternative products such as soy, and almond drinks, and adoption of a
vegan diet. Vitamin D deficiency is very
common in some countries, and over 42% of Singapore residents (Bi etal, 2016),
45.5% of Saudi residents, and in 2018 over 82.5% of females in South Korea (an
increase from 76% in 2008)(Park etal, 2018) were found to be vitamin D
deficient. Vitamin D status decreases with increases in weight (one of the
predisposing maternal factors for giving birth to a child with ASD). In
Australia in 2012, 30% of women of child bearing age were found to be vitamin D
deficient.
Paralleling the rapid rise in the incidence of ASD
in many countries has been the adoption of high SPF value cosmetics in many
countries. Thus, when compared to the early 1990s, where very few "daily"
cosmetics had any SPF protection in them, in 2018 many "daily" cosmetics "boast"
SPF values of 60+ and above. Vitamin D has a critical role in brain development, and even
transient deficiency in the womb can result in delayed brain development (Goh
etal, 2014). Further studies comparing vitamin D sufficient and vitamin D
deficient children showed more autism related traits in those who were born to
mothers with vitamin 25-OH-D concentrations less than 25nmol/L (Napoli etal,
2014). Similarly, lower vitamin D levels in the first trimester of pregnancy
have also been associated with a higher risk of autism (52). It has also been shown that lower vitamin D levels
are common in ASD children than in age matched controls (Parikh etal, 2009; Bi
etal, 2016; Park etal, 2018). Associated with this has been a massive increase
in the rate of vitamin D deficiency diagnosis. Thus, in the period 2000 to 2014
there was an 83-fold increase in the rate of vitamin D deficiency diagnosis in
children in the UK (
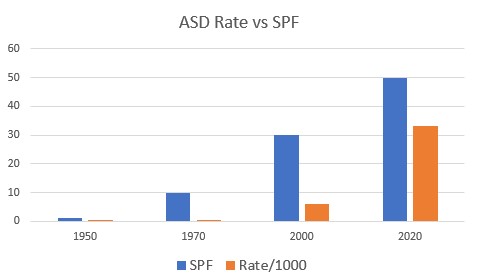
Vitamin D deficiency is common in
Autism
Vitamin D deficiency
and the Development of high SPF cosmetics
Reproduced from Basatemur et al. 2017
Many mothers report very low vitamin D levels during pregnancy and others report extensive use of high SPF cosmetics. Many studies have also found vitamin D deficiency to be common in ASD individuals (Bener 2017), and have suggested that low maternal vitamin D may be a risk factor for the development of ASD, possibly via its action on fetal brain development and altered immune status (Grant 2009). Our own studies have shown a major shift in vitamin D processing associated SNPs (see the page on genetics). The rapid increase in the use of sun-blocks in cosmetics, as well as the increase in SPF values of these products are some of the few associative factors that could account for the increase in rate of the condition. There are few other factors that could account for such an increase, certainly not de novo mutations as has been suggested by some (Kenney 2010).
Children with autism have been found to have lower levels of vitamin D, than their age-matched peers (Stainaker etal, 2019; Bivona etal, 2019: George etal, 2019; Alzqhoul etal, 2019; Chauhan etal, 2019). Studies in animal models have shown that vitamin D deficient animals exhibited delayed motor and behavioural features similar to ASD (Ali etal, 2019). Treatment of ASD children with vitamin D (2000 IU/day) showed significant reduction in their autism rating scales (Feng etal, 2019; Mazahery etal, 2019). In Australia, the effectiveness of the "Slip, Slop, Slap" campaign promoting sun-protection (starting in the late 1980s), was severely criticized as early as 2002 (Nowson etal, 2002), as at that stage the prevalence of vitamin D deficiency in women had already reached 23%, increasing the risk of osteoporosis, dementia, schizophrenia, respiratory condition, diabetes, coronary disease, breast, and prostate cancer. In New Zealand as long ago as 2015, they were claiming the Slip, Slop, Slap campaign had gone too far.More recently the Australan Cancer council has added a warning to their web-site about vitamin D deficiency, thereby absolving themselves of blame.

Paradoxically, since the introduction of the slip, slop, slap campaign in Australia the incidence of melanoma in males has increased over two and one half fold!

Melanoma of the skin statistics | Cancer Australia
Vitamin D Deficiency and Development of Speech
Low vitamin D in utero and in the new-born has been associated with delayed speech development (Hawes etal, 2015).
Vitamin D Deficiency and Epilepsy
Several studies have shown an association between low vitamin D levels and epilepsy in autism (Holló etal, 2014; Miratashi Yazdi etal, 2017; Specht, etal, 2020; Jésus, etal, 2020; Shellhaas, abd Joshi, 2010; Kija, etal, 2019; Elmazny, etal, 2020; Durá-Travé, etal, 2018; Zhang, etal, 2020; Fong, etal, 2020; Snoeijen-Schouwenaars etal, 2015). Epilepsy, intellectual disability and low vitamin D levels were commonly associated (Snoeijen-Schouwenaars etal, 2015)
Vitamin D Deficiency and Iron Deficiency
Several studies have shown an association between low iron and low vitamin D levels, presumably because iron is used in processing of vitamin D (Akermanns etal, 2017; in utero and in the new-born has been associated with delayed speech development (Hawes etal, 2015; Kamau etal, 2018; Malczewska-Lenczowska etal, 2018).
Elevated Urinary Phosphoric Acid in Autism
Active vitamin D is correlated with increased calcium by HMTA, but is inversely correlated with urinary organic acids data. This can be further complicated by diet, as many children with autism have been placed on GFCF diets, with the mistaken belief that the children are intolerant to casein. Much more likely is that functional B12 deficiency results in decreased production of melatonin with the result that the gastrointestinal mucosa does not secrete sufficient lactase and hence the children are actually lactase intolerant. Fixing functional B2 and B12 deficiency is thus essential. Further, putting the child on a dairy-free diet reduces the amount of calcium in their diet, thus resulting in brittle bones, but since calcium is a signaling molecule in the brain, this can be even more detrimental to the child's well-being.

Resolving Vitamin D Deficiency in Pregnant mothers
Current recommendations are a daily intake of at least 800 IU per day, however many studies suggest that a minimum of 1000 IU per day, particularly during pregnancy, and 2000 IU per day for women "at risk", such as those who are over-weight, have darker skin, or who cover-up extensively. Vitamin D deficiency has been defined as less than 50 nmol/L (20 ng/ml). Good sources of vitamin D are milk, or fortified dairy products such as yogurt, butter, margarine, cheese, and fish, such as tuna, mackerel, sardines and salmon. In some areas of the world vitamin D deficiency in pregnant women is so common, that vitamin D testing is no longer done, due to cost, and vitamin D supplementation is strongly recommended. Vitamin D deficiency in mothers has been associated with multiple sclerosis, increased cancer risk, metabolic syndrome, premature delivery, pre-eclampsia, and depression.
Vitamin D deficiency in mothers is becoming increasingly more common, and studies in Japan showing 89.5% of mothers below 20 ng/ml vitamin D (Shibata etal, 2011), while in Greece 19.5% of mothers had levels below 10 ng/ml (Nicolaidou etal, 2006), in Turkey 46.6% below 10 ng/ml (Ustuner etal, 2011), Norway 71% (Viljkainen etal, 2010), whilst in the US 42.4% of African Americans, and 4.2% of whites had serum vitamin D less than 15 ng/ml (Nesby-O'Dell etal, 2002). Despite recommendations few mothers take vitamin D supplements, and only 0.6% of Scottish mothers were found to have taken the recommended supplement dose (Haggarty etal, 2013), nor had neonates in a study in UAE despite recommendations to do so (Narchi etal, 2011).
Resolving Vitamin D Deficiency in Autism
Recent recommendations for vitamin D suggest targeting a minimal level of 40-70 ng/ml 25(OH)D in serum in mothers (Wydert 2014). At least one study has shown a decrease in core symptoms of ASD following vitamin D supplementation of a vitamin D deficient child (Jia 2015).
Changing the paradigm
Unfortunately too many cosmetic companies are earning billions of dollars from the sale of the high SPF cosmetics, so it is highly unlikely that they will change the formulations. It is also unlikely that while so many health professionals are making money out of treating vitamin D associated conditions such as autism, dementia, and Parkinson's disease, that they will change their strategy. Hence "
“It is difficult to get a man to understand something when his salary depends on his not understanding it.” (John Sinclair, 1932). Hence we have been unable to make progress with the cancer council in getting them to stop recommending the use of sun-protection products, nor several cosmetic companies. Hence it is not in the best interests of foundations getting millions of dollars in donations, for them to find a mode of prevention or cure for a condition that they are getting donations for. For instance the Australian Cancer Council (https://www.concer.org.au) has a major arm of its fund raising in selling SPF cosmetics, and clothing. This is despite countless publications on the protective effect of vitamin D and also how elevated vitamin D is protective against UVB mediated damage in the skin (Jagoca and Dixon, 2020; Song etal, 2012; Gupta etal, 2006).Associated Deficiencies in Autism
Low vitamin D levels have been associated with many conditions, including ricketts, PCOS, asthma, multiple sclerosis, atopic dermatitis, cancer risk, metabolic syndrome, poor immunity.
References
-
Goh et al Mitochondrial dysfunction as a neurobiological subtype of Autism Spectrum Disorder.. JAMA psychiatry 2014 71: 665-671
-
Gomez‐Pinedo U, Cuevas JA, Benito‐Martín MS, et al. Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav. 2020;10:e01498 10.1002/brb3.1498 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
-
Napoli et al Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics 2014 133:e1405-10
-
Parikh et al A modern approach to the treatment of mitochondrial disease. Current Treatment Options in Neurology. 2009 11: 414-430
-
Bi et al. 2016 Prevalence of vitamin D deficiency in Singapore: Its implications to Cardiovascular Risk Factors. PMC4723156
-
Park et al. Vitamin D status in South Korean population... Medicine, 2018 97 Issue 26
-
Ali etal 2016 Developmental vitamin D deficiency and autism: Putative pathogenic mechanisms. PMID 28027915
-
Vinkhuyzen etal 2018 Gestational vitamin D deficiency and autism-related traits PMID 27895322
-
Chen etal 2016 Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring PMID 27663117
-
Garipardic etal, 2017 Association of Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorders with mean platelet volume and vitamin D PMID 28319054
-
Ariganjoye, 2017 Pediatric hypovitaminosis D: Molecular perspectives and clinical implications PMID 28229097
-
Endres etal, 2016 Vitamin D deficiency in adult patients with schizophreniform and autism spectrum disorders PMID 27766084
-
Cannell and Grant 2013 What is the role of vitamin D in autism?. PMID 24494055
-
Grant and Soles 2009 Epidemiologic evidence supporting the role of maternal vitamin D as a risk factor for the development of infantile autism PMID 20592795
-
Kenney etal 2010 Environmental risk factors for autism: de they help cause de novo genetic mutations that contribute to the disorder. PMID 19699591
-
Stainaker etal, 2019 Rickets treatment improves more than bone health in toddler with autism spectrum disorder..PMID 31452890
-
Bivona etal, 2019 Vitamin D and the nervous system PMID 31142227
-
Chauhan etal 2019 Vitamin D deficiency in children with psychiatric illness in a tertiary care hospital in North India PMC 30765965
-
Ali etal 2018 Developmental vitamin D deficiency produces behavioural phenotypes of relevance to autism in the animal model. PMC 31137843
-
George etal, 2019 Prevalence and risk factors of hypovitaminosis-d in children with cognitive and movement disorders. PMC 31020592
-
Feng et al. 2019 Clinical effect of vitamin D3 combined .... in the treatment of autism spectrum disorder in toddlers PMC 31014425
-
Alzqhoul etal, 2019 The association between serum vitamin D3 levels and autism among Jordanian Boys PMC 30993503
-
Mazahery etal, 2019 A randomised controlled trial of vitamin D...... autism spectrum disorder PMC 30744880
-
Duan, etal 2013 Relationship between vitamin D and autism spectrum disorder PMID 23965890
-
Weydert 2014 Vitamin D in children's health PMID 27417476
-
Cannell 2013 Autism, will vitamin D treat core symptoms? PMID 23725905
-
DeLuca etal 2013 Review: the role of vitamin D in nervous system health and disease PMID 23336971
-
Jia etal, 2015 Core symptoms of autism improved after vitamin D supplementation PMID 25511123
-
Kesby etal 2009 Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain PMID 19500655
-
Jiang etal 2014 Neurochemical effects of chronic administration of calcitriol in rats PMID 25533012
-
Pertile etal, 2016 Vitamin D signaling and the differentiation of developing dopamine systems PMID 27450565
-
Basatemur et al. Trends in the diagnosis of vitamin D deficiency Pediatrics 2017 139 PMC5337117
-
Shibata et al. High prevalence of hypovitaminosis D in pregnant Japanese women with threatened premature delivery. J Bone Miner Metab 2011 29:615-20 PMID21384110
-
Nicolaidou et al. Low vitamin D status in mother-newborn pairs in Greece. Calcif Tissue Int 2006 78:337-42 PMID16830197
-
Haggarty etal. Vitamin D in pregnancy at high latitude in Scotland. Br J. Nutr 2013 109:898-905
-
Narchi etal, Longitudinal study of vitamin D status in the 1st 6 months of life. Ann Trop Paediatr. 2011 31:225-30
-
Ustuner etal. Maternal serum 25(OH)D levels in the third trimester of pregnancy during the winter season. Matern Fetal Neonatl Med 2011 24:1421-6
-
Viljakainen etal. Maternal vitamin D status determines bone variables in newborns. Endocrinol Metab. 2010 95: 1749-57
-
LowNesby-O'Dell etal Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age... Am J Clin Nutr. 2002 76: 1987-92
-
Geller AC, Clapp RW, Sober AJ, Gonsalves L, Mueller L, Christiansen CL, Shaikh W, Miller DR. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013 Nov 20;31(33):4172-8. doi: 10.1200/JCO.2012.47.3728. Epub 2013 Sep 16. PMID: 24043747; PMCID: PMC3906570.
-
Nowson etal, Med J Aust 2002; 177: 149–52
-
Jésus, P., Godet, B., Darthou-Pouchard, L., Fayemendy, P., Abdallah-Lebeau, F., Villeneuve, O., Marcon, C., Gimenez, L., Preux, P. M., Couratier, P., & Desport, J. C. (2020). Vitamin D status among patients with drug-resistant and non-drug-resistant epilepsy. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition, 90(3-4), 205–209. https://doi.org/10.1024/0300-9831/a000459
-
Shellhaas, R. A., & Joshi, S. M. (2010). Vitamin D and bone health among children with epilepsy. Pediatric neurology, 42(6), 385–393. https://doi.org/10.1016/j.pediatrneurol.2009.12.005
Kija, E., Gidal, B. E., Shapson-Coe, A., Cader, S., van der Watt, G., Delport, S., & Wilmshurst, J. M. (2019). Vitamin D abnormalities and bone turn over analysis in children with epilepsy in the Western Cape of South Africa. Seizure, 69, 186–192. https://doi.org/10.1016/j.seizure.2019.04.020 -
Elmazny, A., Amer, H., Rashed, L., Khalil, S., & Magdy, R. (2020). Vitamin D status of untreated children and adolescent Egyptian patients with genetic generalized epilepsy: A case-control study. Epilepsy & behavior : E&B, 103(Pt A), 106840. https://doi.org/10.1016/j.yebeh.2019.106840
-
Durá-Travé, T., Gallinas-Victoriano, F., Malumbres-Chacón, M., Moreno-Gónzalez, P., Aguilera-Albesa, S., & Yoldi-Petri, M. E. (2018). Vitamin D deficiency in children with epilepsy taking valproate and levetiracetam as monotherapy. Epilepsy research, 139, 80–84. https://doi.org/10.1016/j.eplepsyres.2017.11.013
-
Zhang, X., Liu, Z., Xia, L., Gao, J., Xu, F., Chen, H., Du, Y., & Wang, W. (2020). Clinical features of vitamin D deficiency in children: A retrospective analysis. The Journal of steroid biochemistry and molecular biology, 196, 105491. https://doi.org/10.1016/j.jsbmb.2019.105491
-
Fong, C. Y., Kong, A. N., Poh, B. K., Mohamed, A. R., Khoo, T. B., Ng, R. L., Noordin, M., Nadarajaw, T., & Ong, L. C. (2016). Vitamin D deficiency and its risk factors in Malaysian children with epilepsy. Epilepsia, 57(8), 1271–1279. https://doi.org/10.1111/epi.13443
-
Snoeijen-Schouwenaars, F. M., van Deursen, K. C., Tan, I. Y., Verschuure, P., & Majoie, M. H. (2015). Vitamin D supplementation in children with epilepsy and intellectual disability. Pediatric neurology, 52(2), 160–164. https://doi.org/10.1016/j.pediatrneurol.2014.10.001
-
Best CM, Riley DV, Laha TJ, Pflaum H, Zelnick LR, Hsu S, Thummel KE, Foster-Schubert KE, Kuzma JN, Cromer G, Larson I, Hagman DK, Heshelman K, Kratz M, de Boer IH, Hoofnagle AN. Vitamin D in human serum and adipose tissue after supplementation. Am J Clin Nutr. 2020 Nov 12;113(1):83–91. doi: 10.1093/ajcn/nqaa295. Epub ahead of print. PMID: 33184642; PMCID: PMC7779222.
-
Michie C. Managing vitamin D deficiency in children. London Journal of primary care July 2010 londonjournalofprimarycare.org.uk/articles/3186685.pdf
-
NHS Choices. nhs.uk/news/2012/01January/Pages/vitamin-d-medical-advice-and-supplements.aspx
-
Prevention of rickets and vitamin D deficiency in Birmingham: the case for universal supplementation. infantfeedingwm.org.uk/documents/Website_VitD.pdf
-
SACN Update on Vitamin D 2007. sacn.gov.uk/pdfs/sacn_position_vitamin_d_2007_05_07.pdf
-
SACN Vitamin D and Health 2016 www.gov.uk/government/uploads/system/uploads/attachment_data/file/537616/SACN_Vitamin_D_and_Health_report.pdf
-
https://www.scientificamerican.com/article/vitamin-d-deficiency-united-states/
-
Vitamin D (nih.gov) https://www.ncbi.nlm.nih.gov/books/NBK500914/pdf/Bookshelf_NBK500914.pdf
-
Madsen KH, Rasmussen LB, Andersen R, Mølgaard C, Jakobsen J, Bjerrum PJ, Andersen EW, Mejborn H, Tetens I. Randomized controlled trial of the effects of vitamin D–fortified milk and bread on serum 25-hydroxyvitamin D concentrations in families in Denmark during winter: the VitmaD study. Am J Clin Nutr. 2013 Aug;98(2):374-82. doi: 10.3945/ajcn.113.059469. PMID: 23783292.
Jagoda SV, Dixon KM. Protective effects of 1,25 dihydroxyvitamin D3 and its analogs on ultraviolet radiation-induced oxidative stress: a review. Redox Rep. 2020 Dec;25(1):11-16. doi: 10.1080/13510002.2020.1731261. PMID: 32093585; PMCID: PMC7054951.
Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, Reeve VE, Mason RS. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007 Mar;127(3):707-15. doi: 10.1038/sj.jid.5700597. Epub 2006 Dec 14. PMID: 17170736.
Song EJ, Gordon-Thomson C, Cole L, Stern H, Halliday GM, Damian DL, Reeve VE, Mason RS. 1α,25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J Steroid Biochem Mol Biol. 2013 Jul;136:131-8. doi: 10.1016/j.jsbmb.2012.11.003. Epub 2012 Nov 16. PMID: 23165145.
Nowson etal, Med J Aust 2002; 177: 149–52
Russell-Jones, GJ. Altered Metabolism of Iron, Vitamin B, and Vitamin B12 Potentially Interfere with Vitamin D- Activation in Children with Autism. J Med - Clin Res & Rev. 2024a; 8(5): 1-6.
Russell-Jones GJ. Complex interaction between iron, vitamin B2, vitamin B12 and vitamin D during the activation of vitamin D. J Med - Clin Res & Rev. 2024b; 8(6); 1-5
-
Copyright © 2018 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited