Vitamin B12 Deficiency in Autism
-
Vitamin B12 loading of the brain happens predominantly in the womb, with little other vitamin B12 loading of the brain for the rest of life
-
Vitamin B12 loading of the brain increases progressively as the foetus matures.
-
Premature babies have lower brain vitamin B12.
-
Vitamin B12 deficiency during pregnancy leads to vitamin B12 deficiency in the neonate
-
Vitamin B12 deficiency during pregnancy increases the risk for preterm labour, low birth weight and increased infant mortality.
-
The brains of children with autism have been found to have greatly reduced levels of vitamin B12.
-
Vitamin B12 deficiency leads to lower production of creatine, and that alone can give most of the symptoms of autism
-
All children with ASD have been found to be functionally deficient in vitamin B12 at time of assessment
-
Vitamin B12 deficiency in the neonate is associated with delayed physical and mental development.
-
Vitamin B12 deficiency in the mothers during pregnancy is known to cause severe retardation of myelination of the nervous system of the foetus.
-
Vitamin B12 deficiency during development is associated with delay in the development of speech
-
Inadequate myelination in the various regions of the brain is common in children with autism
-
Vitamin B12 deficiency reduces the production of melatonin in the child and is associated with sleep disorders in ASD
-
Vitamin B12 deficiency has been associated with epilepsy in children with ASD
-
Paradoxical B12 deficiency is common in children with ASD
Vitamin B12 Deficiency in Neonates
Infants born with cobalamin (vitamin B12) deficiency are at significant risk of lasting brain damage. Further, the deficiency can cause developmental and intellectual delay, hypotonia, tremor, seizure, and failure to thrive. In addition the children may have speech, linguistics and social impairments, as well as behavioural disorders, and problems with fine and gross motor movement. Without therapy, there can be irreversible intellectual impairment, as well as cognitive and developmental delay Hasbaoui etal, 2021. Of these the concurrence of hypotonia with developmental and intellectual delay, especially with premature birth, low birth weight, difficulties feeding, and problems sleeping are all "Red Flags" for Vitamin B12 deficiency. They are also all associated with autism. It is almost unbelievable that despite countless publications on the effects of vitamin B12 deficiency in the neonate that this association with autism is missed by the medical profession, who do not test for metabolic makers of vitamin B12 deficiency, such as homocysteine, and MMA (.
Vitamin B12 Deficiency and Hypotonia
Identification of hypotonia in neonates is a strong indication of potential vitamin B12 deficiency (either absolute or paradoxical) Chalouhi et al, 2008; Demir et al, 2013; Bousselamti et al, 2018;Acıpayam et al, 2020; Akcaboy etal, 2015; Serin et al, 2019; Incecik et al, 2010; Honzik et al, 2010; Bicakci 2015; Smolka etal, 2001;Taskesen et al, 2011; Gupta et al, 2019; Benbir etal, 2007; Vieira etal, 2020; Ma etal, 2011; Borkowska etal 2007; Wagnon etal, 2005; Kamoun etal, 2017; Tosun etal, 2011; Kose etal, 2020; Lövblad etal, 1997; Lücke etal, 2007; Hall 1990; Vieira etal, 2020; Taskesen etal, 2011; Serin etal, 2015; Bicakci 2015; Serin HM, Arslan , 2019; Aguirre etal, 2019; Casella etal, 2005; Acıpayam etal, 020; Bousselamti etal, 2018;Hasbaoui etal, 2021 Hypotonia, is very common in autism, and early diagnosis of autism should be suspected in children with hypotonia, as "Hypotonia is a recognizable marker of ASD and should serve as a "red flag" to prompt earlier recognition and neurodevelopmental evaluation toward an autism diagnosis." (Gabis etal 2021; Lopez-Espejo, etal, 2021). Hypotonia is associated with decreased language development and IQ in autism (Osljeskova etal, 2007; Fillano etal, 2002). Not surprisingly hypotonia is a common symptom in those with autism (Badescu et al, 2016; Oslejskova et al, 2007; Lopez-Espejo et al, 2021; Gabis et al, 2021). Whilst the authors of the aforementioned papers did not come to any conclusion about the reason for vitamin B12 deficiency and hypotonia, clearly in methyl B12 deficiency there is reduced production of creatine, due to the reduced activity of GNMT (Longo etal, 2011; Pacheva etal, 2016; Stöckler et al, 1994; Mercimek-Mahmutoglu et al, 2006; Stockler-Ipsiroglu et al, 2014; Mercimek-Mahmutoglu et al 2014; O'Rourke et al, 2009; Araújo et al, 2005; Lion-François et al, 2006; Mercimek-Mahmutoglu et al, 2009; Leuzzi et al, 2013 Schulze et al, 2006;Verbruggen et al 2007; Morris et al, 2007; Item etal, 2004), and reduced production of CoQ10, both of which would lead to poor muscle tone. Several studies have shown a link between creatine deficiency and hypothonia (Longo etal, 2011), including studies on deficiency of the creatine producing enzyme Guanidoacetate-N-methyl transferase (Nasrallah et al, 2012); and the creatine transporter (Yıldız etal, 2020;
Pacheva etal, 2016; Morris etal, 2007; Schulze 2013). Developmental delay was an accompanying symptom.Vitamin B12 Loading of the Foetal Brain
It is known that the majority of vitamin B12 loading of the brain occurs during foetal development where as much as 17% of transplacentally derived vitamin B12 enters the foetal brain. Loading is maximal during the last trimester of foetal life, and continues until the time of birth and thereafter very, very little enters the brain (Roed etal, 2008: Agarwal and Nathani, 2009
). As such foetal loading of the brain is incredibly important for the developing child, and deficiency of vitamin B12 in the mothers has a profound effect on the foetus and new-born child. Deficiency of vitamin B12 in the mothers is also correlated with deficiency of vitamin B12 in the neonate. An alarming rate of vitamin B12 deficiency in pregnant mothers in the UK has recently been reported (Sukumar etal, 206; Knight etal, 2015; Low-Beer etal, 1968), with more that 20% of women deficient as assessed by the haematological definition of deficiency (<150 pmol/L), but a massive 70% being deficient if assessed by metabolic parameters (<250 pmol/L; Sukumar etal, 206; Knight etal, 2015; Low-Beer etal, 1968). It would appear that the rates may have been dropping for some time, because in 1968 (before UK joined the EU), the average B12 levels were much higher at 288 pmol/L (Low-Beer etal, 1968) The rates of deficiency were much higher in India, where 43% were deficient (<150 pmol/L; Krishnaveni etal, 2009). Hopefully this is not a portend of ever increasing rates of autism. Additionally, despite the diagnosis of B12 deficiency, the mothers in the various studies were not treated for B12 deficiency! The incidence of B12 deficiency in pregnancy seems to be very high, with over 50% of woman in Canada being metabolically deficient in the first trimester (Roed etal, 2008). The increase in the adoption of vegan diets will potentially result in a dramatic increase in the rate of both vitamin B12 deficiency and that associated iron deficiency (Lemale etal, 2019), and the associated developmental delay in the upcoming pediatric population. Lower levels of vitamin B12 have been found in the brains of children with autism (Zhang etal, 2016).

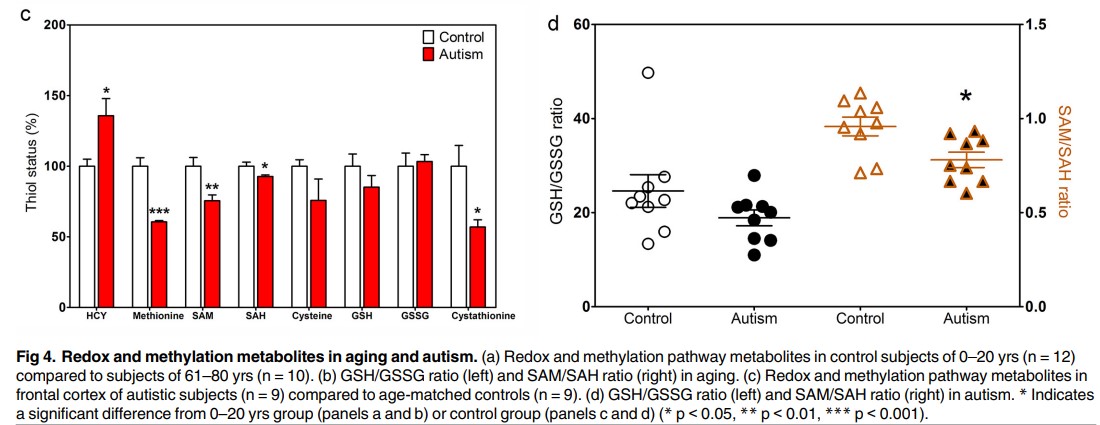
In addition, there is increased homocysteine, and reduced levels of methionine, SAM and lower thiol reducing activity with lower Cysteine, and GSH. Of particular note is the lower level of cystathionine, the initial product of CBS through its reduced action on homocysteine, suggesting a block methylation and in conversion of Hcy to Cystathioinine.
Vitamin B12 Deficiency and Creatine deficiency
Over 40% of all methylation within the brain goes to the production of creatine, an essential energy transporter in muscles and brain. As the level of methyl B12 decreases, so too does the formation of creatine. Creatine deficiency has been associated with severe neurodevelopmental delay, intellectual disability, behavioral abnormalities, poorly developed muscle mass and muscle weakness (Stockebrand etal, 2018; Braissant etal, 2011). Creatine deficiency has also been associated with epilepsy and aphasia (difficulty reading, speaking and writing - a common problem in children with autism)(Perna etal, 2016), and with mental retardation, autism, hypotonia, and seizures (Longo etal, 2011). Creatine deficiency has been shown to reduce energy transfer from the electron transport chain (in the mitochondria) to energy available within the cytoplasm of the cell. (Nabuurs etal, 2013). Creatine deficiency has also been shown to affect spatial and object learning (Udobi etal, 2019), Creatine deficiency has also been associated with conditions such as Huntington's, ALS, Parkinson's disease, and Chronic Fatigue Syndrome (Riesberg etal, 2016). Creatine plays an essential role in myelination of neuronal cells by the oligodendrocytes, which use them for energy. In low creatine, there is poor myelination and developmental delay results (Rosko et al 2021). Creatine also has an important role in remyelination, and as such deficiency in creatine, or functional B2/B12 will result in poor remyelination and ultimately lead to myelin breakdown.
Vitamin B12 Deficiency and Developmental Delay
For over 60 years it has been known that Vitamin B12 sufficiency is crucial for the development of myelination of the central nervous system, and poor vitamin B12 status is linked to poor growth and neurodevelopment (Gutierrez-Diaz, 1959; Schrimshaw etal, 1959; Agrawal and Nathani 2009; Sheng etal, 2019
), neural tube defects (Lucke etal 2007), and retardation of myelination in the brain (Lovblad etal, 1997; Horstmann etal, 2003), and lower brain volume (Black 2008). Vitamin B12 deficiency is associated with severe brain atrophy with signs of retarded myelination, with the frontal and temporal lobes being the most severely affected (Lövblad et al,1997). The frontal lobes are involved in motor functions, problem solving, memory, language, judgement, impulse control, spontaneity and social and sexual behaviour. The temporal lobes are involved in the formation of long term memory, recognizing faces, and interpreting body language, it aids in the production of speech, remembering the names of objects, and recognition of language. These are the levels of highest creatine usage with creatine having a role in a range of cognitive functions, including learning, memory, attention, speech and language, and possibly emotion. Thus, vitamin B12 deficiency in the mothers, which is later seen in the children, would be expected to have adverse outcomes. Further, maternal vitamin B12 status early in gestation (28 weeks) has been positively associated with child's subsequent mental and social development quotients, as measured at 2 years (Strand etal 2018). Vitamin B12 concentration in the first 2 years of life was positively correlated with cognitive score (Sheng etal, 2019). Infants aged 12-18 months who have lower B12 levels also present with lower psychomotor and mental development scores compared to those with higher vitamin B12 levels (Obeid etal, 2017). This would "fit" with the critical time for foetal brain loading of the child (Agrawal and Nathany, 2009; Chalouhi etal, 2008), and vitamin B12 and folate deficiency, with the accompanying elevated homocysteine have been associated with altered brain morphology, and cognitive and psychological problems in school-aged children (Ars etal, 2019) . Furthermore, vitamin B12 deficiency, particularly of methyl B12, results in lower production of the methylating agent, S-Adenosylmethionine (SAM). Lower SAM in turn leads to lower energy production of creatine (the essential backbone for creatine-phosphate) and ubiquinol (CoQ10), the essential electron transfer molecule in the Electron Transport Chain. Low CoQ10 levels have been associated with lower cognitive function and intellectual disability in autism (Smolka etal, 2001). Reduced production of SAM also affects the activity of the histamine-neutralizing enzyme, Histamine-N-methyl transferase, and would explain much of the food insensitivity of young children with ASD, due to the presence of histamine in a diverse range of foods.Vitamin B12 and the Production of Melatonin
Melatonin, together with vitamin D, stimulates neuronal stem cells to differentiate into oligodendrocytes, which are the cells in the brain that are responsible for myelination of the nerves in the brain. Production of melatonin gradually increases during pregnancy, peaking in the third trimester. After birth, the newborn child initially relies on melatonin in the mother's milk, as it gradually turns on its own production of melatonin, which in neurotypically normal children peaks at around 5 years of age, and starts to decline after puberty. It has been known for over 60 years, that the production of melatonin involves the O-methylation of N-acetyl serotonin, by the action of enzyme hydroxyindole-O-methyl transferase, using S-Adenosylmethionine (SAM), as the methyl donor (Axelrod and Weissbach 1960, Weissbach and Axelrod 1960). As such production of melatonin, ultimately relies on methyl cobalamin as the initial methyl donor for the production of SAM, and so in mothers that are low in vitamin B12, foetal melatonin will be lower, as too will neonatal melatonin, thereby resulting in the delayed myelination typical of ASD. Despite the obvious correlation between low functional vitamin B12 resulting in a reduced ability to produce melatonin, we could find very little evidence that this association has been made in the literature. This is despite countless publications, finding an association between lower melatonin production in the mother, the fetus, or in the neonate, and the severity of symptoms in autism (Wiebe etal, 2018; Yunho etal, 2018; Gagnon and Godbout, 2018; Rossignol and Frye, 2011; 2014, Sanchez-Barcelo et al, 2017; Haidar etal 2016). Further, rather than to measure and address the vitamin B12 deficiency in such children, melatonin is the more common treatment (Blackmer and Feinstein, 2016). Further, the association was still not made in studies showing the elevated melatonin precursor, N-acetylserotonin, and reduced melatonin in ASD (Pagan etal, 2014).
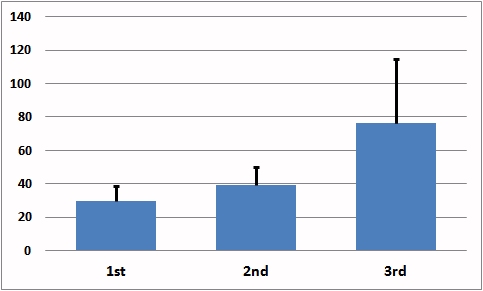
Melatonin levels in mothers in the 1st, 2nd, and 3rd Trimester
Voiculescu etal, 2014
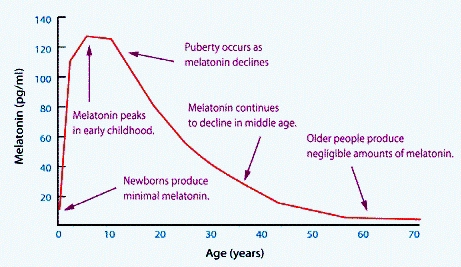
Melatonin levels during developmentr
Grivas and Savvidou, 2007
The final step in production of Melatonin is the methylation of N-Acetyl-Serotonin (NAcSer) by the enzyme HydroxyIndole-O-methyltransferase (HIOMT), which has an absolute requirement for S-Adenosylmethionine (SAM), a product of the methylation cycle (Axelrod and Weissbach 1960, Weissbach and Axelrod 1960).
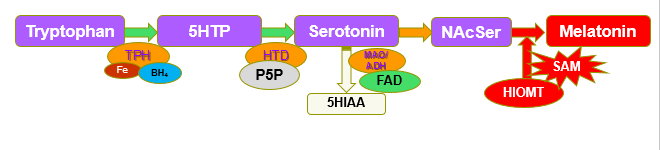
Melatonin synthesis and SAM
In Methyl B12 deficiency, there is a greatly reduced production of SAM, and breakdown products of tryptophan, Kynurenic acid (KA) and Quinolinic acid (QA), as well as the breakdown product of Serotonin, 5-Hydroxyindoleacetic acid (5HIAA) start to accumulate and can be detected as elevated levels in urine.
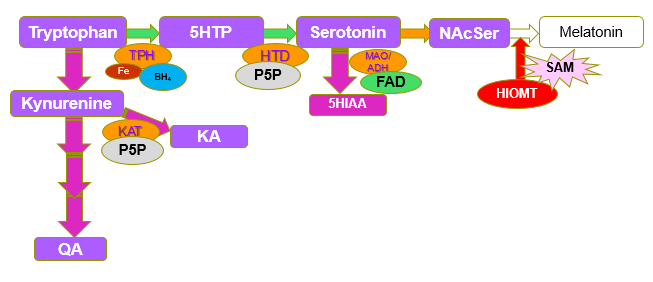
Metabolites increased in SAM deficiency
In functional B2 deficiency due to lack of Iodine and/or Selenium, riboflavin is not converted to FMN and then levels of serotonin and KA are reduced.

The typical symptoms of vitamin B12 deficiency in the neonate are very similar to those observed in autism and include megaloblastic anemia, feeding difficulties, developmental delay (Casella etal, 2005; Honzik etal, 2010; Hall 1990), microcephaly (Honzik etal, 2010; Hall 1990), failure to thrive, hypotonia (Aquirre etal, 2019; Casella etal, 2005; Kanra etal, 2005; Chandra etal, 2006; Lucke etal, 2007; Schlapbach etal, 2007; Borkowska etal, 2007; Honzik etal, 2010; Hall 1990), and cerebral atrophy with symptoms of lethargy (Hall 1990; Shevell and Rosenblat 1992), and occasionally seizures (
Benbir etal, 2007;Aquirre etal, 2019; Hall 1990), and psycho-motor delay. Seizures may also occur during treatment for B12 deficiency, however, these go away within days or weeks (Benbir etal, 2007) Many of these symptoms can be explained by the critical role that vitamin B12 plays in the production of melatonin, through its role in methylation. Melatonin in turn is critical for the differentiation of neuronal stem cells into myelin-producing oligodendrocytes, potentially explaining the delayed myelination found in children with autism.Adenosyl Vitamin B12 Deficiency
A deficiency in the Adenosyl-form of vitamin B12 has been linked to tiredness, vomiting, weak muscle tone, developmental delay, intellectual disability, and frequent illnesses. In functional B2 deficiency, the child has reduced capacity to gain energy from fats, as the reductase is FAD-dependent, or to gain energy from sugar, due to the need of pyruvate decarboxylase for TPP, lipoate and FAD. Hence the body turns to the metabolism of protein for energy. The break-down of proteins results in increased levels of the 9 essential amino acids lysine, tyrosine, phenylalanine, tryptophan, methionine, and the branched chain amino acids leucine, isoleucine, and valine. Of these lysine, tyrosine, phenylalanine and tryptophan cannot be processed for energy as their break-down products enter the glycolysis pathway and so cannot be used, thus energy must be obtained from methionine, and the branched chain amino acids (BCA acids). Processing of the later requires MMA-CoA mutase an Adenosyl-B12 dependent enzyme. In Adenosyl-B12 deficiency, levels of urinary methyl malonic acid are elevated. Elevated BCA acids are found in autism (Gao et al, 2024)
Other markers of Adenosyl B12 deficiency include ethyl malonic acid, and methyl succinic acid. Methylsuccinate is a by-product of the metabolism of methionine and threonine. Ethylmalonic acid and methylsuccinic acid are altered metabolites of isoleucine (Nowaczyk et al, 1998). Elevated ethylmalonicacid and methylsuccinic acid have been associated with developmental delay, hypotonia, and vascular instability associated with lactic acidemia (Nowaczyk et al, 1998). Functional vitamin B2 deficiency, also results in the catabolism of hydroxyproline, leading to elevated oxalate, pyruvate, hippuric acid, glycolate, and glyoxylate. Elevated levels of MMA, EMA, MSA, oxalate, hippuric acid, glycolate and glyoxylate are common in autism. Conversely, levels of methionine, leucine, cysteine, threonine are lower in ASD (Bala etal, 2016; Li et al, 2018).
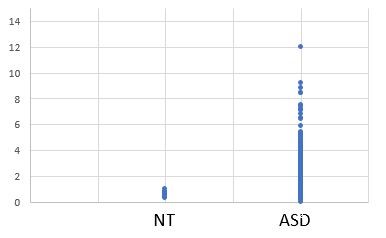
Methyl malonic acid in urine of children with autism.
Vitamin B12 Deficiency in Vegetarian Mothers
Children born of vegan and vegetarian mothers often have moderate to severe vitamin B12 deficiency
, and such deficiencies have been associated with delayed myelination, weight loss, and reduction of motor skills, delayed development, neuro-regression, regression of psychomotor development, growth retardation, neuropathy (Renault etal, 1999) brain atrophy and apathy (Davis and Melina 2014; Kanra etal, 2005; Stollhoff and Schulte 1987; Von Schenck et al, 1997). Many of these conditions persist through later life (von Schenck etal, 1997), and even with supplementation after birth, children can still show apathy, muscular dystonia, abnormal movements and language delay (Smolka etal 2001). Despite these deficiencies being well documented, for more than 30 years, many vegetarian and vegan mothers do not supplement before, during or after pregnancy, nor do their health professionals check them for deficiency.Accompanying the vitamin B12 deficiency of the vegan and vegetarian diets are deficiencies in protein, calcium, iron, zinc, and omega-3 fatty acids (97-98-99), so much so that the German Nutrition Society does NOT recommend such diets during pregnancy, lactation, and childhood (99).
Maternal serum B12 levels are closely correlated with the vitamin B12 levels in the mother's milk. In the years 2009 to 2017, there was an increase in the rate of veganism in the US from 0.1% to 6%, and in increase in the rate of autism from 1:200 to 1:35 over the same period.
Vitamin B12 and the Development of Speech
Myelination of Brocca's region in the brain precedes the development of speech, and as such delayed myelination would be expected to cause the delay in speech which is so characteristic of many children with autism.
Vitamin B12 deficiency and Depression
Depression is a common side-effect of vitamin B12 deficiency, and can lead to thoughts of, and commitment of, suicide in children with autism
Vitamin B12 deficiency and Nitrous oxide and anaesthetics.
Use of Nitrous oxide either as an anaesthetic or though inhalation from a "Nang" can have disastrous affects on the function of vitamin B12. During the methylation reaction of MethylCo(III)B12 + Homocysteine, the product, Co(I)B12 + Methionine is formed. In the absence of 5MTHF, free Co(I)B12 can readily reacts with nitrous oxide to form NO-Co(III)B12, which is inactive, yet will “clog up” methylation by Methionine synthase, and irreversibly inactivate the enzyme, hence explaining the toxicity of Nitrous oxide..
Higher levels of Co(I)B12 are present in functional B2 deficiency, due to lack of activity of MTHFR, particularly with those mutations in the MTHFR protein , or in those with a diet low in folate, thereby making those individuals more susceptible to the action of Nitrous oxide. The inactive NO-Co(III)B12 would be indistinguishable from inactive Co(II) B12, and when measured in the current total serum B12 and the inappropriately named active B12 tests, as they do not distinguish which analogue of cobalamin is being measured, cyanocobalamin, hydroxycobalamin, methylcobalamin, adenosylcobalamin, Co(II)cobalamin, Co(I)cobalamin, glutathionyl-Co(III)cobalamin or NO-Co(III)cobalamin, to name but a few. The extent of damage that nitrous can do to the nervous system can be gleaned from those who use Nangs, and their devastating neurological consequences. Reports of side-effects include “subacute-onset, progressive distal lower limb sensory symptoms and unsteadiness”, “subacute combined degeneration of the cord”” ataxia and progressive paresis”, depression, development of diseases of the brain, spine and nerves. The severity of these reactions has led the UK government to consider criminalizing the use of Nitrous Oxide.
Nitrous oxide was commonly used as an anaesthetic gas, yet as long ago as 1956 (Lassen et al, 1956) it was realized that it the activity of vitamin B12 was destroyed by nitrous oxide and could cause megaloblastic anemia. In 1968, Banks and co-workers demonstrated that nitrous oxide could react with the cobalt in vitamin B12 and lead to the inactive NO-CoB12 complex. The destruction of the activity of vitamin B12 is dependent upon the time and dose of administration of nitrous, with over 50% of individuals producing signs of megaloblastic depression of bone marrow function (Nunn and Chanarin, 1978). As early as 1978 (Amess et al, 1987) the use of nitrous oxide for anaesthesia was found to be contra-indicated, yet to this day it is still used, and many individuals report signs of B12 deficiency following use. Unbelievably, despite numerous publications showing poor outcomes of nitrous oxide use in pregnancy, and several demonstrating an association between nitrous and autism, and over 200 publications, demonstrating inactivation of vitamin B12 with subsequent sequelae, clinicains in the US, UK and Australia claim "“ Initiation and management of nitrous oxide by registered nurses is a safe and cost-effective option for labor pain.”. (See PDF). One of the problems with Nitrous inactivation of vitamin B12 activity is that the levels of B12 in serum still remain high, yet paradoxically the B12 is inactive - as per the discussion on paradoxical vitamin B12 deficiency. Unbelievably, nitrous oxide is still used as an anaesthetic to this day in the USA and Australia, both on mothers during pregnancy, and also on young children. Evidence suggests that this alone is responsible for many cases of autism (Xin et al, 2024). It has been known for over 40 years that the use of nitrous oxide in anaesthesia (laughing gas) or in recreational abuse, can cause vitamin B12 deficiency (Shah and Murphy, 2019: Tani etal, 2019; Oussalah etal, 2019; Chi, 2018; Stockton etal, 2017; Massey etal, 2016: Garakani etal, 2014; Safari etal, 2013; Chiang etal, 2013; Krajewski etal, 2007; Cohen etal, 2007; Jameson etal, 1999; Smith, 2001: Deleu etal, 2001; Mayall, 1999; Horne and Holloway, 1997: Kinsella and Green 1995; Carmel etal, 1993; Koblin etal,1990; O'Leary etal, 1985; van der Westhuyzen and Metz, 1984; 1982; Lumb etal, 1982; Kondo etal, 1981: Seteinberg etal, 1981; McKenna etal, 1980; Linnell etal, 1978; Deacon etal, 1978). Post surgical complications of the use of Nitrous include peripheral neuropathy (Neuveu etal, 2019: Egan, 2018: Kaski etal, 2017; Richardson 2010), metabolic encephalopathy (Vive etal, 2019), myeloneuropathy (Edigin etal, 2019; Friedlander and Davies, 2018; Alt etal, 2011; Waklawik etal, 2003; Sesso etal, 1999: Nestor and Stark, 1996), neuropathy (Gullestrup etal, 2019; Conaerts etal, 2017:Middleton and Roffers, 2018), pancytopenia (Norris and Mallia, 2019), Myopathy (Williamson etal, 2019), myelopathy (Dong etal, 2019; Mancke etal, 2016; Probasco etal, 2011: Hathout and El-Saden, 2011; Pema et al, 1998), severe neuropsychiatric symptoms (Lundin etal, 2019), combined degeneration of the spinal chord (Lan etal, 2019; Patel etal, 2018; Anderson etal, 2018; Antonucci, 2018; Keddie etal, 2018; El-sadawi etal, 2018; Yuan etal 2017: Buizert etal, 2017; Chen and Huang, 2016; Pugliese etal, 2015: Chaugny etal, 2014; Cheng etal, 2013; Lin etal, 2011; Wijesekera, etal, 2009; Renaud etal, 2009: Wu etal, 2007; Ahn and Brown, 2005 Ilniczky etal, 2003: Beltramello etal, 1998: Rosener and DIchgans, 1996), neurotoxicity (Johnsonn etal, 2018), neuronopathy (Morris etal, 2015), polyneuropathy (Alarcia etal, 1999), psychosis (Sethi etal, 2006), dementia (El Otmani etal, 2007), ataxia (Miller etal, 2004), megaloblastic anemia (Barbosa etal, 2000), neurological impairment (McNeeely etal, 2000), neurologic decompensation (Felmet etal, 2000), neurologic degeneration (Flippo and Holder, 1993), spastic paraparesis (Lee etal, 1999). Curiously, Nitrous is still recommended by the American Association of Anesthesiologists, NSW Department of Health, and the Association of Anesthesiologists, the New Zealand College of Midwives.. In fact, several countries with high standards of healthcare, such as Canada, Sweden, Australia, Finland, and the United Kingdom, use a blend of 50% oxygen and 50% nitrous oxide to treat pain during labor.They do, though, express concerns about the potential effect on Global warming, which is of greater concern that the effect on the neonatal brain!! The rational appears to be due to the replacement of epidural medication, with its risk on the spine, with the nitrous oxide. This attitude typifies the medical profession, treat the problem now, worry about the side effects later. We have contacted numerous hospitals, the Royal Children's Hospital Melbourne, Mayo Clinic Kopabirth, NZ College of Midwives, midwife associations, The America Pregnancy Association, Queensland Government, Doctors for the Environment and anaesthesiologists expressing our concerns yet not one has "returned our call". Atrocious!! Interestingly, the increase in the use of Nitrous from around 1% of births in 1980 to now 35=45% of births in 2024, has paralleled the rise in the rate of autism from <0.1% to now ~ 3%.
Determination of vitamin B12 Deficiency
Simplistically one would assume that simply measuring vitamin B12 levels in serum would determine if a person was sufficient or insufficient, and to a large extent this is what is done. Most Pathology labs simply measure the amount of B12 in serum and using an arbitrary cut-off value (generally 150 pmol/L) assign values above this as being sufficient. Unfortunately it is nowhere near that simple. Even in common dietary insufficiency, signs of biochemical deficiency of vitamin B12 can be observed when vitamin B12 levels drop below 250 pmol/L.
Measurement of biochemical deficiency has uncovered a huge range of serum B12 levels even as high as 2000 pmol/L in which biochemical deficiency of vitamin B12 can be measured. This, then is paradoxical and the term "Paradoxical vitamin B12 deficiency" has been used to describe this condition. It appears that in "paradoxical B12 deficiency", the form of B12 that is in serum is an inactive form of B12 (most likely to be Co(II)B12). If this form of B12 was present in the mother during pregnancy it would be this form of B12 (the inactive Co(II)B12) that would have stocked the brain, with the result that the child would be born with what seems to be adequate vitamin B12 levels, however, the child would be functionally deficient in vitamin B12. Further, the B12 in breast milk from the mother would also be inactive. Paradoxical B12 deficiency is common in children with ASD (Hope etal, 2020). Studies by Dr Russell-Jones have shown that every child with ASD was functionally deficient in vitamin B12, with the majority also having Paradoxical B12 deficiency.
Thus, the only way to tell if the vitamin B12 in serum is active or inactive is to measure metabolic by-products of B12 metabolism and see if they are raised. The two most commonly raised markers in vitamin B12 deficiency are homocysteine and methyl malonic acid (MMA). There are a number of others that are readily identified if an assessment of urinary Organic Acids is performed. Interpretation of such data should though only be attempted by those sufficiently trained in such assessment, which the general medical profession are not. Elevated homocysteine is common in children with autism (Kałużna-Czaplińska, etal, 2011; Altun etal, 2018)
Markers associated with Vitamin B12 Deficiency
SAM:SAH ratio As vitamin B12 deficiency increases lack of methyl transferase activity leads to elevations in Homocysteine, and a decrease in the ratio of SAM:SAH
GSH:GSSG ratio. Reduced methylation causes a reduction in the transfer of the sulphur from homocysteine into the sulphation cycle, leading to lower intracellular cysteine, and reduced production of glutathione. Lack of cysteine then causes an increase in Pyroglutamic acid, one of the surrogate markers for vitamin B12 deficiency. Reduced GSH works in combination with thiosulfate sulphur transferase in the formation of SeCystRNA, and the efficacy of thereaction drops in functional B12 deficiency. In addition levels of toxic intracellular sulphite increase (ASD 107 nmol/ml, NT 2.1 nmol/ml) as well as thiosulfate (ASD 131 nmol/ml, NT 19 nmol/ml) (Kruithof et al, 2020). This can then result in a metabolic spiral, as lack of production of SeCystRNA, will reduce the production of Selenoproteins, such as the deiodinases that are responsible for conversion of T4 to T3. This in turn leads to lower production of ribofavin kinase, with a reduced activity of MTHFR and MTRR, which are critical for maintaining the activity of MethylB12.
Resolving Vitamin B12 Deficiency in Pregnant mothers
Mothers should ensure vitamin B12 sufficiency before they are pregnant, however, if this is not possible, urinary Organic Acids Testing should be carried out to establish sufficiency, and cases of deficiency mothers should supplement not only with vitamin B12, but also with Iodine, Selenium, Molybdenum and vitamin B2 if there is reason to believe that these may also be deficient. Warning signs in the mothers can be fatigue, obesity, gestational diabetes, insufficient dietary intake such as occurs in vegetarian or vegan diets. Correcting of deficiency cannot be achieved by large oral doses of vitamin B12 due to both the very limited uptake of vitamin B12 from the gut, as well as the extensive denaturation of the majority of the orally administered dose of vitamin B12, by gastric acid. Instead vitamin B12 should be given by injection or via the TransdermoilTM delivery route. Any person on antidepressant medication going into or during pregnancy should suspect vitamin B12 or iron deficiency, and get checked via OAT.
Resolving Vitamin B12 Deficiency in Autism
Vitamin B12 deficiency has been shown to occur in all children with ASD and this needs to be addressed if the child is going to have any chance of normal development. Several studies on children who were vitamin B12 deficient have shown significant increase in growth and cognitive scores when supplemented with vitamin B12 (Sheng etal, 2019; Strand etal, 2015). Given that co-deficiency in functional vitamin B2 is universal in autistic children this deficiency must be fixed first, and then the active forms of vitamin B12, adenosyl B12 and methyl B12 must be given either by injection of via the TransdermoilTM delivery route
. NO oral formulation of vitamin B12 has ever been shown to resolve symptoms in autism.Other signs of Vitamin B12 Deficiency in Neonates
Other signs of vitamin B12 deficiency in the neonate include megalobastic anaemia, feeding difficulties (difficulties in suckling), developmental delay, microcephaly, hyptonia, lethargy, irritability, involuntary movements, seizures and cerebral atrophy" (Benbir etal, 2007).
Associated Deficiencies in Autism
The majority of studies looking at vitamin B12 deficiency in children and in autism have now addressed the likely co-deficiency of iron, however, one could assume that a diet low in vitamin B12 would also be a diet low in iron. Every child that we have data for who has autism is also deficient in active vitamin B2 (FMN and FAD) and is deficient in active vitamin B12 (Adenosyl and Methyl B12), these deficiencies also have to be addressed or the child will not progress developmentally. Accompanying these deficiencies, deficiencies of Iodine, Selenium and/or Molybdenum are very common.
Resolving Vitamin B12 Deficiency in the Brain
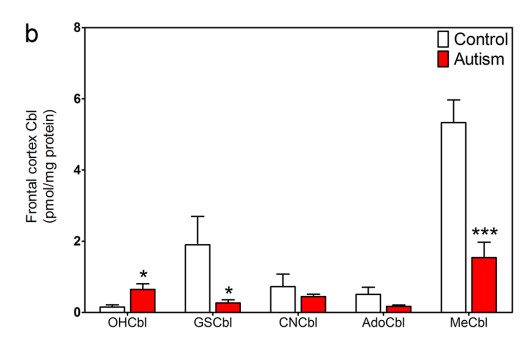
Transport of vitamin B12 into the brain happens primarily during the last trimester of pregnancy. Once this has occurred, the brain becomes almost recalcitrant to further uptake of vitamin B12, and seems to have to survive on what was in the brain at the time of birth. This can be seen in levels of vitamin B12 detected in the brains of subjects with normal serum B12 levels as they age (Zhang et al, 2016). Of particularly note is the huge drop in both Methyl and Adenosyl B12 in the Frontal Cortex in those over 61..
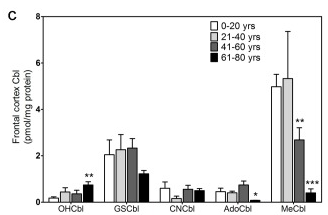
Attempts to resolve this deficiency through intravenous administration are hindered by the very limited amount of vitamin B12 taken into the brain following even intravenous administration, as can be seen in numerous imaging studies.

As can be seen in the study by Flodh (1967), the brain of the mouse seems to have virtually no uptake of 131-I-cobalamin.
Calculation of 89Zr-Cobalamin PET Tracer (Workinger, et al, 2017), confirmed these findings, and showed almost no detectable uptake into the brain.
The corollary to this is that if, the brain is loaded with "dud" B12 in utero, or if the brain is exposed to vitamin B12 modifying agents such as Nitrous Oxide, it will be almost impossible to displace the alterred vitamin B12.
Copyright.
The descriptions and findings on vitamin B12 and autism, is the property of B12 Oils Pty Ltd. Reproduction in whole or in part constitutes an infringement in the Copyright law. Copyright infringement carries serious penalties.
References
-
Black 2011 Effects of vitamin B12 and folate deficiency on brain development in children PMC3137939
-
Jain etal 2015 Vitamin B12 deficiency in children: a treatable cause of developmental delay 24453156
-
Hall CA. Function of vitamin B12 in the central nervous system as revealed by congenital defects. Am J Hematol. 1990 Jun;34(2):121-7. doi: 10.1002/ajh.2830340208. PMID: 1692663.
-
Dubaj C, Czyż K, Furmaga-Jabłońska W. Vitamin B12 deficiency as a cause of severe neurological symptoms in breast fed infant - a case report. Ital J Pediatr. 2020 Mar 30;46(1):40. doi: 10.1186/s13052-020-0804-x. PMID: 32228659; PMCID: PMC7106665.
-
Blom H. Methylmalonic acid values in healthy Dutch children. Eur J Nutr. 2008 Feb;47(1):26-31. doi: 10.1007/s00394-007-0692-5. Epub 2007 Dec 18. PMID: 18092123.
-
Mariani A, Chalies S, Jeziorski E, Ludwig C, Lalande M, Rodičre M. Conséquences de l'allaitement maternel exclusif chez le nouveau-né de mčre végétalienne--A propos d'un cas [Consequences of exclusive breast-feeding in vegan mother newborn--case report]. Arch Pediatr. 2009 Nov;16(11):1461-3. French. doi: 10.1016/j.arcped.2009.07.027. Epub 2009 Sep 11. PMID: 19748244.
-
Baatenburg de Jong A, Bekhof J, Zwart P, Langenhorst VJ, Roorda RJ. Ontwikkelingsachterstand bij borstgevoede kinderen door ontoereikend dieet van de moeder [Developmental delay in breastfed children due to inadequate diet of the mother]. Ned Tijdschr Geneeskd. 2006 Mar 4;150(9):465-9. Dutch. PMID: 16553042.
-
Dorsvik I, Ueland PM, Markestad T, Bjřrke-Monsen AL. Cobalamin supplementation improves motor development and regurgitations in infants: results from a randomized intervention study. Am J Clin Nutr. 2013 Nov;98(5):1233-40. doi: 10.3945/ajcn.113.061549. Epub 2013 Sep 11. PMID: 24025626.
-
Öztürk Z, Arhan E, Aydin K, Hirfanoğlu T, Tümer L, Okur I, Serdaroğlu A, Akbaş Y, Karaoğlu B. COBALAMIN C DEFICIENCY WITH INFANTILE SPASM AND CUTANEOUS FINDINGS: A UNIQUE CASE. Genet Couns. 2016;27(3):399-403. PMID: 30204970.
-
Wang F, Han L, Yang Y, Gu X, Ye J, Qiu W, Zhang H, Zhang Y, Gao X, Wang Y. Clinical, biochemical, and molecular analysis of combined methylmalonic acidemia and hyperhomocysteinemia (cblC type) in China. J Inherit Metab Dis. 2010 Dec;33 Suppl 3:S435-42. doi: 10.1007/s10545-010-9217-0. Epub 2010 Oct 6. PMID: 20924684.
-
Gowda VK, Srinivasan VM. A Treatable Cause of Global Developmental Delay with Autism Spectrum Disorder Due to Cobalamin Related Remethylation Disorder. Indian J Pediatr. 2022 Aug;89(8):832. doi: 10.1007/s12098-022-04221-0. Epub 2022 May 23. PMID: 35604587.
-
Zengin E, Sarper N, Caki Kiliç S. Clinical manifestations of infants with nutritional vitamin B deficiency due to maternal dietary deficiency. Acta Paediatr. 2009 Jan;98(1):98-102. doi: 10.1111/j.1651-2227.2008.01059.x. Epub 2008 Oct 6. PMID: 18945280.
-
El Din EMS, Rabah TM, Metwally AM, Nassar MS, Elabd MA, Shalaan A, Kandeel W, El Etreby LA, Shaaban SY. Potential Risk Factors of Developmental Cognitive Delay in the First Two Years of Life. Open Access Maced J Med Sci. 2019 Jun 30;7(12):2024-2030. doi: 10.3889/oamjms.2019.566. PMID: 31406549; PMCID: PMC6684437.
-
Tanc C, Yildiz I. Evaluation of Neurodevelopmental Screening Test Scores in Children with Vitamin B12 Deficiency. Neuropediatrics. 2024 Apr;55(2):97-103. doi: 10.1055/s-0043-1777125. Epub 2023 Dec 20. PMID: 38122810.
-
Bravo J P, Ibarra C J, Paredes M M. Compromiso neurológico y hematológico por déficit de vitamina B12 en lactante hijo de madre vegetariana: caso clínico [Hematological and neurological compromise due to vitamin B12 deficit in infant of a vegetarian mother: case report]. Rev Chil Pediatr. 2014 Jun;85(3):337-43. Spanish. doi: 10.4067/S0370-41062014000300010. PMID: 25697251.
-
Blom H. Methylmalonic acid values in healthy Dutch children. Eur J Nutr. 2008 Feb;47(1):26-31. doi: 10.1007/s00394-007-0692-5. Epub 2007 Dec 18. PMID: 18092123.
-
Bousselamti A, El Hasbaoui B, Echahdi H, Krouile Y. Psychomotor regression due to vitamin B12 deficiency. Pan Afr Med J. 2018 Jun 20;30:152. doi: 10.11604/pamj.2018.30.152.12046. PMID: 30374398; PMCID: PMC6201603.
-
Acıpayam C, Güneş H, Güngör O, İpek S, Sarışık N, Demir NŞ. Cerebral atrophy in 21 hypotonic infants with severe vitamin B12 deficiency. J Paediatr Child Health. 2020 May;56(5):751-756. doi: 10.1111/jpc.14733. Epub 2019 Dec 23. PMID: 31868292.
-
Chalouhi C, Faesch S, Anthoine-Milhomme MC, Fulla Y, Dulac O, Chéron G. Neurological consequences of vitamin B12 deficiency and its treatment. Pediatr Emerg Care. 2008 Aug;24(8):538-41. doi: 10.1097/PEC.0b013e318180ff32. PMID: 18708898.
-
Bjřrke-Monsen AL, Ueland PM. Cobalamin status in children. J Inherit Metab Dis. 2011 Feb;34(1):111-9. doi: 10.1007/s10545-010-9119-1. Epub 2010 May 27. PMID: 20508991.
-
JADHAV M, WEBB JK, VAISHNAVA S, BAKER SJ. Vitamin B12 deficiency in Indian infants. A clinical syndrome. Lancet. 1962 Nov 3;2(7262):903-7. doi: 10.1016/s0140-6736(62)90682-7. PMID: 13964414.
-
Doyle JJ, Langevin AM, Zipursky A. Nutritional vitamin B12 deficiency in infancy: three case reports and a review of the literature. Pediatr Hematol Oncol. 1989;6(2):161-72. doi: 10.3109/08880018909034282. PMID: 2702070.
-
Graham SM, Arvela OM, Wise GA. Long-term neurologic consequences of nutritional vitamin B12 deficiency in infants. J Pediatr. 1992 Nov;121(5 Pt 1):710-4. doi: 10.1016/s0022-3476(05)81897-9. PMID: 1432418.
-
Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev. 2008 May;66(5):250-5. doi: 10.1111/j.1753-4887.2008.00031.x. PMID: 18454811.
-
Honzik T, Adamovicova M, Smolka V, Magner M, Hruba E, Zeman J. Clinical presentation and metabolic consequences in 40 breastfed infants with nutritional vitamin B12 deficiency--what have we learned? Eur J Paediatr Neurol. 2010 Nov;14(6):488-95. doi: 10.1016/j.ejpn.2009.12.003. Epub 2010 Jan 20. PMID: 20089427.
-
Ganesan S, Thanawala N, Hussain N. Vitamin B12 deficiency: a treatable cause of developmental delay in infancy. J Paediatr Child Health. 2013 Apr;49(4):E348-9. doi: 10.1111/jpc.12158. PMID: 23574564.
-
Hawes D, Shute PE, Dicke O, Paul SP. Vitamin B12 deficiency presenting with solid food aversion and global developmental delay in a child. Br J Hosp Med (Lond). 2014 Jun;75(6):352-3. doi: 10.12968/hmed.2014.75.6.352. PMID: 25040415.
-
Akcaboy M, Malbora B, Zorlu P, Altınel E, Oguz MM, Senel S. Vitamin B12 Deficiency in Infants. Indian J Pediatr. 2015 Jul;82(7):619-24. doi: 10.1007/s12098-015-1725-3. Epub 2015 Apr 5. PMID: 25840526.
-
Azad C, Jat KR, Kaur J, Guglani V, Palta A, Tiwari A, Bansal D. Vitamin B12 status and neurodevelopmental delay in Indian infants: a hospital-based cross-sectional study. Paediatr Int Child Health. 2020 May;40(2):78-84. doi: 10.1080/20469047.2019.1638130. Epub 2019 Jul 3. PMID: 31267850.
-
Lin YC, Chung CJ, Huang YL, Hsieh RL, Huang PT, Wu MY, Ao PL, Shiue HS, Huang SR, Su CT, Lin MI, Mu SC, Hsueh YM. Association of plasma folate, vitamin B12 levels, and arsenic methylation capacity with developmental delay in preschool children in Taiwan. Arch Toxicol. 2019 Sep;93(9):2535-2544. doi: 10.1007/s00204-019-02540-4. Epub 2019 Aug 31. PMID: 31473767.
-
Warghat PA, Sharath HV, Raghuveer R. The Effect of Early Pediatric Rehabilitation in an Infant With Vitamin B12 Deficiency Associated With Developmental Delay: A Case Report. Cureus. 2024 Jun 18;16(6):e62648. doi: 10.7759/cureus.62648. PMID: 39036156; PMCID: PMC11258930.
-
Dupuy G, Roux CJ, Barrois R, Imbard A, Pontoizeau C, Dangles MT, Aubart M, Arnoux JB, Margoses D, Brassier A, Marbach C, Bérat CM, Sarda E, Gitiaux C, de Lonlay P, Boddaert N, Schiff M, Desguerre I. Vitamin deficiencies in children: Lessons from clinical and neuroimaging findings. Eur J Paediatr Neurol. 2024 May;50:6-15. doi: 10.1016/j.ejpn.2024.02.013. Epub 2024 Feb 26. PMID: 38520815.
-
Mütze U, Gleich F, Haas D, Urschitz MS, Röschinger W, Janzen N, Hoffmann GF, Garbade SF, Syrbe S, Kölker S. Vitamin B12 Deficiency Newborn Screening. Pediatrics. 2024 Aug 1;154(2):e2023064809. doi: 10.1542/peds.2023-064809. PMID: 39040028.
-
Jain etal 2015 Vitamin B12 deficiency in children: a treatable cause of developmental delay 24453156
-
Rasmussen etal, 2001 Vitamin B12 deficiency in children and adolescents S0022
-
Sukumar etal, 2016 Vitamin B12 status among pregnant women in the UK and its association with obesity and gestational diabetes. PMID 27916927
-
Knight etal 2015 Lower circulating B12 is associated with higher obesity and insulin resistance during pregnancy in a non-diabetic white British Population. PMID
-
Low-Beer, etal, 1968 Serum vitamin B12 levels and vitamin B12 binding capacity in pregnant and non-pregnant Europeans and West Indians
-
Krishnaveni etal, 2009 Low plasma vitamin B12 in pregnancy is associated with gestational 'diabeity' and later diabetes. PMID 19707742
-
Lai etal, 2017 High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes PMID: 28381340
-
Zhang etal, 2016 Decreased levels of vitamin B12 in aging, autism and schizophrenia. PMID: 26799654
-
Chu etal, 2016 Effects of melatonin and its analogues on neural stem cells. PMID 26499359
-
Rudnitskaya etal, 2015 Melatonin Attenuates Memory Impairment, Amyloid-β Accumulation, and Neurodegeneration in a Rat Model of Sporadic Alzheimer's Disease.
-
Shen etal, 2016 Effect of Melatonin and Resveratrol against Memory Impairment and Hippocampal Damage in a Rat Model of Vascular Dementia. PMID 28419991
-
Li etal, 2017 Effect of Melatonin on renewal of chick small intestinal mucosa PMID 28431176
-
Whiton etal, 1979 Brain damage in infancy and dietary B12 deficiency PMID 502936
-
Smolka etal, 2001 Metabolic complications and neurological manifestations of vitamin B12 deficiency in children of vegetarian mothers. PMID 11787236
-
Zengin etal, 2008 Clinical manifestations of infants with nutritional vitamin B deficiency due to maternal dietary deficiency PMID 18945280
-
Halicioglu etal, 2011 Nutritional deficiency in infants of vitamin B12 deficient mothers PMID 99429203
-
Demir etal, 2013 Clinical and neurological findings of severe vitamin B12 deficiency... PMID 23781950
-
Kvestad etal Vitamin B12 status in infancy is positively associated with development and cognitive functioning 5 y later in Nepalese children. Am. J. Clin Nut. 2017 105(5):1122-1131
-
Strand etal, The effect of vitamin B12 supplementation in Nepalese infants...Trials, 2017: 187
-
Strand etal, Maternal and infant vitamin B12 status during infancy predict linear growth at 5 yrs, Ped Res 2018 https://doi.org/10.1038/s41390-018-0072-2
-
Smith, AD 2018 Maternal and infant vitamin B12 status and development. Ped Res. https://doi.org/10.1038/s41390-018-0110-0
-
Guez etal, 2012 Severe vitamin B12 deficiency in an exclusively breastfed 5-month-old Italian infant born to a mother receiving multivitamin supplementation during pregnancy PMC
-
Roed etal, 2008 Severe vitamin B12 deficiency in infants breastfed by vegans (Ugesr Laeger, 171: 3099-101
-
Agrawal and Nathani, 2009 Neruo-regression in vitamin B12 deficiency MBJ 2009
-
Gutierrez-Diaz, 1959 Effect of magnesium, molybdate, vitamin B12 and vitamin T complex, alone and combined, on development in children. Act Ped Esp. 17; 125-53
-
Riesberg LA, Weed SA, McDonald TL, Eckerson JM, Drescher KM. Beyond muscles: The untapped potential of creatine. Int Immunopharmacol. 2016 Aug;37:31-42. doi: 10.1016/j.intimp.2015.12.034. Epub 2016 Jan 8. PMID: 26778152; PMCID: PMC4915971.
-
Rosko L, Gentile T, Smith V, Huang JK. 86583 The role of creatine in developmental myelination and remyelination. J Clin Transl Sci. 2021 Mar 30;5(Suppl 1):99. doi: 10.1017/cts.2021.655. PMCID: PMC8827867.
-
Schimshaw etal, 1959 Growth and development of Central American children. II The effect of oral administration of vitamin B12 to rural children of preschool and school age. Am J. Clin Nutr. 7: 180-4
-
Chalouhi et al, 2008 Neurological consequences of vitamin B12 deficiency and its treatment. Ped Emerg Care, 24: 538-41
-
Sheng etal, 2019 Effects of dietary intervention on vitamin B12 status and cognitive level of 18 month-old toddlers in high poverty areas...BMC Pert 19:334
-
Smolka etal 2001 Metabolic complications and neurological manifestations of vitamin B12 deficiency in children of vegetarian mothers. Cas Lek Cesk 140: 732-5
-
Lucke et al, 2007 Maternal vitamin B12 deficiency: cause for neurological symptoms in infancy. Z Geburtshilfe Neonatol, 211: 157-161
-
Obeid etal, 2017 Cobalamin status from pregnancy to early childhood.... Adv Nutr 8: 971-979
-
Lovblad etal 1997 Retardation of myelination due to dietary vitamin B12 deficiency: cranial MRI findings Pediatr Radiol 27: 155-8
-
Horstemann etal 2003 Infantile cobalamin deficiency with cerebral lactate accumulation and sustained choline depletion. Neuroped 34; 261-4
-
Rosenblatt etal, 1985 Prenatal vitamin B12 therapy of a fetus with methylcobalamin deficiency. Lancet, 18: 1127-9
-
Graham etal, 1992 Long-term neurologic consequences of nutritional vitamin B12 deficiency in infants. J Ped. 121:710-4
-
Stockebrand etal, 2018 A mouse model of creatine transporter deficiency reveals impaired motor function and muscle energy metabolism. Front Physiol 9, 773
-
Braissant etal. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amion Acids, 2011 40; 1315-24
-
Perna etal Creatine transporter deficiency leads to increased whole body and cellular metabolism. Amino Aicds 2016 48; 2057-65
-
Udobi eatl, Deletion of the creatine transporter gene in neonatal, but not adult, mice leads to cognitive defects J Inherit Metab Dis. 2019; 42; 966-974
-
Renault etal 1999 Neuropathy in two cobalamin-deficient breast-fed infants of vegetarian mothers. Muscle Nerv 22:252-4
-
Nabuurs etal, Disturbed energy metabolism and muscular dystrophy caused by pure creatine deficiency are reversible by creatine intake. J Physiol. 2013 591;571-92
-
Longo etal. Disorders of creatine transport and metabolism. Am J Med Genet C Semin Med Genet 2011 157; 72-8
-
Hall CA 1990 Function of vitamin B12 in the central nervous system as revealed by congenital defects. Am J Hematol. 34:121-7
-
Kosenen and Pihko 1994 Development regression in a child caused by vitamin B12 deficiency. Duodecim. 110: 588-91
-
Monfort-Gouraud et al. 1993 Severe megaloblastic anemia in a child breast fed by a vegetarian mother. Ann Pediatr 40,:28-31
-
von Schenck etal, 1997 Persistence of neurological damage induced by dietary vitamin B12 deficiency in infancy. Arch Dis. Child, 77: 137-9
-
Tashiro etal, 1983 Phosphatidylethanolamine methyltransferase activity in developing, demyelinating, and diabetic mouse brains. Tohuku. J. Exp. Med 141 S485-90
-
Axelrod and Weissbach 1960 Science, 131, 1312
-
Weissbach and Axelrod 1960 J. Fed Proc. 19, 50
-
Weibe etal. Low maternal melatonin level increases Autism Spectrum Disorder Risk in children. Res Deve Disabil 2018 92, 79-89
-
Yunho et al. The relationship between autism spectrum disorder and melatonin during fetal development. Molecules 2018, 23,
-
Gagnon and Godbout Melatonin and comorbidities in children with autism spectrum disorder. Curr Dev Disord Rep 2018, 5, 197-206
-
Rossignol and Frye Melatonin in Autism Spectrum Disorders: a systematic review and meta-analysis. Dev Med Child Neurol, 2011, 53, 783-792
-
Rossignol and Frye Melatonin in Autism Spectrum Disorders. Curr Clin Pharmacol 2014 9, 326-34
-
Sanchez-Barcelo et al. Clinical uses of Melatonin in neurological diseases and mental and behavioural disorders Curr Med Chem, 2017 24, 3851-3878
-
Haidar etal. Low oxytocin and melatonin levels and their possible role in the diagnosis and prognosis in Iraqi Autistic children. Saudi Med J. 2016 37, 29-36
-
Blackmer and Feinstein Management of sleep disorders in children with neurodevelopmental disorders: A review Pharmacotherapy. 2016, 36, 84-98
-
Pagan, et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for Autism Spectrum Disorders. Trans Psychiatry, 2014 4, e479
-
Voiculescu et al. Role of melatonin in embryo fetal development. J Med Life. 2014;7(4):488–492
-
Grivas and Savvidou Melatonin the "light of night"...Scoliosis 2007; 2, 6
-
Hasbaoui BE, Mebrouk N, Saghir S, Yajouri AE, Abilkassem R, Agadr A. Vitamin B12 deficiency: case report and review of literature. Pan Afr Med J. 2021 Mar 4;38:237. doi: 10.11604/pamj.2021.38.237.20967. PMID: 34046142; PMCID: PMC8140678.
-
Bousselamti A, El Hasbaoui B, Echahdi H, Krouile Y. Psychomotor regression due to vitamin B12 deficiency. Pan Afr Med J. 2018 Jun 20;30:152. doi: 10.11604/pamj.2018.30.152.12046. PMID: 30374398; PMCID: PMC6201603.
-
Acıpayam C, Güneş H, Güngör O, İpek S, Sarışık N, Demir NŞ. Cerebral atrophy in 21 hypotonic infants with severe vitamin B12 deficiency. J Paediatr Child Health. 2020 May;56(5):751-756. doi: 10.1111/jpc.14733. Epub 2019 Dec 23. PMID: 31868292.
-
Casella EB, Valente M, de Navarro JM, Kok F. Vitamin B12 deficiency in infancy as a cause of developmental regression. Brain Dev. 2005 Dec;27(8):592-4. doi: 10.1016/j.braindev.2005.02.005. PMID: 16310594.
-
Aguirre JA, Donato ML, Buscio M, Ceballos V, Armeno M, Aizpurúa L, Arpí L. Compromiso neurológico grave por déficit de vitamina B12 en lactantes hijos de madres veganas y vegetarianas [Serious neurological compromise due to vitamin B12 deficiency in infants of vegan and vegetarian mothers]. Arch Argent Pediatr. 2019 Aug 1;117(4):e420-e424. Spanish. doi: 10.5546/aap.2019.e420. PMID: 31339288.
-
Serin HM, Arslan EA. Neurological symptoms of vitamin B12 deficiency: analysis of pediatric patients. Acta Clin Croat. 2019 Jun;58(2):295-302. doi: 10.20471/acc.2019.58.02.13. PMID: 31819326; PMCID: PMC6884369.
-
Bicakci Z. Growth retardation, general hypotonia, and loss of acquired neuromotor skills in the infants of mothers with cobalamin deficiency and the possible role of succinyl-CoA and glycine in the pathogenesis. Medicine (Baltimore). 2015 Mar;94(9):e584. doi: 10.1097/MD.0000000000000584. PMID: 25738478; PMCID: PMC4553967.
-
Serin HM, Kara AO, Oğuz B. West syndrome due to vitamin B12 deficiency. Turk Pediatri Ars. 2015 Dec 1;50(4):251-3.
-
Taskesen M, Yaramis A, Pirinccioglu AG, Ekici F. Cranial magnetic resonance imaging findings of nutritional vitamin B12 deficiency in 15 hypotonic infants. Eur J Paediatr Neurol. 2012 May;16(3):266-70. doi: 10.1016/j.ejpn.2011.08.005. Epub 2011 Sep 7. PMID: 21903432.
-
Vieira D, Florindo C, Tavares de Almeida I, Macário MC. Adult-onset methylenetetrahydrofolate reductase deficiency. BMJ Case Rep. 2020 Mar 10;13(3):e232241. doi: 10.1136/bcr-2019-232241. PMID: 32161077; PMCID: PMC7066602.
-
Casella EB, Valente M, de Navarro JM, Kok F. Vitamin B12 deficiency in infancy as a cause of developmental regression. Brain Dev. 2005 Dec;27(8):592-4. doi: 10.1016/j.braindev.2005.02.005. PMID: 16310594.
-
Hall CA. Function of vitamin B12 in the central nervous system as revealed by congenital defects. Am J Hematol. 1990 Jun;34(2):121-7. doi: 10.1002/ajh.2830340208. PMID: 1692663.
-
Lücke T, Korenke GC, Poggenburg I, Bentele KH, Das AM, Hartmann H. Mütterlicher Vitamin-B12-Mangel: Ursache neurologischer Symptomatik im Säuglingsalter [Maternal vitamin B12 deficiency: cause for neurological symptoms in infancy]. Z Geburtshilfe Neonatol. 2007 Aug;211(4):157-61. German. doi: 10.1055/s-2007-981249. PMID: 17729202.
-
Lövblad K, Ramelli G, Remonda L, Nirkko AC, Ozdoba C, Schroth G. Retardation of myelination due to dietary vitamin B12 deficiency: cranial MRI findings. Pediatr Radiol. 1997 Feb;27(2):155-8. doi: 10.1007/s002470050090. PMID: 9028851.
-
Tosun A, Aral YZ, Çeçen E, Aydoğdu A, Çetinkaya Çakmak B. Involuntary movement in infants during vitamin B12 treatment. Turk J Haematol. 2011 Dec 5;28(4):317-22. English. doi: 10.5152/tjh.2011.18. PMID: 27264590.
-
Kamoun F, Guirat R, Megdich F, Ben Ameur S, Kallel C, Hachicha M. Frequent Infections, Hypotonia, and Anemia in a Breastfed Infant. J Pediatr Hematol Oncol. 2017 Mar;39(2):141-142. doi: 10.1097/MPH.0000000000000725. PMID: 28060111.
-
Borkowska A, Plata-Nazar K, Łuczak G, Matheisel A. Niedobór witaminy B12 u rocznego dziecka karmionego wyłacznie piersia [Vitamin B12 deficiency in a one-year-old, exclusively breast fed child]. Med Wieku Rozwoj. 2007 Oct-Dec;11(4):435-8. Polish. PMID: 18605198.
-
Longo N, Ardon O, Vanzo R, Schwartz E, Pasquali M. Disorders of creatine transport and metabolism. Am J Med Genet C Semin Med Genet. 2011 Feb 15;157C(1):72-8. doi: 10.1002/ajmg.c.30292. Epub 2011 Feb 9. PMID: 21308988
-
Nasrallah F, Kraoua I, Joncquel-Chevalier Curt M, Bout MA, Taieb SH, Feki M, Khouja N, Briand G, Kaabachi N. Guanidinoacetate methyltransferase (GAMT) deficiency in two Tunisian siblings: clinical and biochemical features. Clin Lab. 2012;58(5-6):427-32. PMID: 22783571.
-
Yıldız Y, Göçmen R, Yaramış A, Coşkun T, Haliloğlu G. Creatine Transporter Deficiency Presenting as Autism Spectrum Disorder. Pediatrics. 2020 Nov;146(5):e20193460. doi: 10.1542/peds.2019-3460. PMID: 33093139.
-
Pacheva I, Ivanov I, Penkov M, Kancheva D, Jordanova A, Ivanova M. Creatine Deficiency Syndrome could be Missed Easily: A Case Report of Guanidinoacetate Methyltransferase Deficiency Presented with Neurodevelopmental Delay, Seizures, and Behavioral Changes, but Normal Structural MRI. Ann Clin Lab Sci. 2016 Sep;46(5):557-61. PMID: 27650626.
-
Morris AA, Appleton RE, Power B, Isherwood DM, Abernethy LJ, Taylor RW, Turnbull DM, Verhoeven NM, Salomons GS, Jakobs C. Guanidinoacetate methyltransferase deficiency masquerading as a mitochondrial encephalopathy. J Inherit Metab Dis. 2007 Feb;30(1):100. doi: 10.1007/s10545-006-0478-2. Epub 2006 Dec 14. PMID: 17171576.
-
Schulze A. Creatine deficiency syndromes. Handb Clin Neurol. 2013;113:1837-43. doi: 10.1016/B978-0-444-59565-2.00053-8. PMID: 23622406
-
Casella etal, 2005 Vitamin B12 deficiency in infancy as a cause of developmental reqression. Brain Dev. 27: 592-4
-
Aquirre etal, 2019 Serious neurological compromise due to vitamin B12 deficiency in infants of vegan and vegetarian mothers. Arch Argent Pediatr 117
-
Ars etal 2019 Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes. Br J. Nutr. 122: S1-S9
-
Black 2008 Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr. Bull. 29: S126-31
-
Renault etal, 1999 Neuropathy in two coblamin-deficient breast-fed infants of vegetarian mothers. Muscle Nerve. 22: 252-4
-
Kanra etal, 2005 Answer to hypotonia: a simple hemogram. J Child Neurol 20: 930-1
-
Stollhoff and Schulte 1987 Vitamin B12 and brain development. Eur J. Pediatr 146: 201-5
-
von Schenck etal 1997 Persistence of neurological damage induced by dietary vitamin B12 deficiency in infancy. Arch Dis Chil 77: 137-9
-
Chandra etal, 2006 Tremors and thrombocytosis during treatment of megaloblastic anaemia. Ann Trop Paediatr 26: 101-5
-
Lucke etal, 2007 Maternal vitamin B12 deficiency: cause for neurological symptoms in infancy. Z Gebrurtshilfe Neonatoal 211: 157-61
-
Schlapbach etal, 2007 Floppy baby with macrocytic anemia and vegan mother. Praxis 29: 1309-14
-
Borkowska etal 2007 Vitamin B12 deficiency in a one-year-old, exclusively breast fed child. Med Wieku Rozwoj 11:435-8
-
Chalouhi etal, 2008 Neurological consequences of vitamin B12 deficiency and its treatment. Pediatr Emerg Care 24:538-41
-
Honzik etal, 2010 Clinical presentation and metabolic consequences of 40 breastfed infants with nutritional vitamin B12 deficiency - what have we learned? Eur J Paediatr Neurol 14:488-95
-
Hall 1990 Function of vitamin B12 in the central nevous system as revealed by congenital defects. Am J. Hematol. 34; 121-7
-
Shevell and Rosenblatt 1992 The neurology of cobalamin. Can J Neurol Sci 19: 472-86
-
Strucinska 2002 Vegetarian diets of breastfeeding women in the light of dietary recommendations. Rocz Panstw Zakl Hig 53: 65-79
-
Rendle-Short et al 1979 Vegan mothers with vitamin B12 deficiency. Med J Aust 3:483
-
Michaud et al, 1992 Nutritional vitamin B12 deficiency: two cases detected by routine newborn urinary screening. Eur J Pediatr. 151;218-20
-
Specker 1994 Nutritional concerns of lactating women consuming vegetarian diets. Am J Clin Nutr. 59: 1182S
-
Renault et al 1999 Neuropathy in two cobalamin-deficient breast-fed infants of vegetarian mother. Muscle Nerv 22:252-4
-
Ueland and Monsen 2003 Hyperhomocysteinemia and B-vitamin deficiences in infants and children. Clin Chem Lab Med. 41:1418-26
-
Weiss et al, 2004 Severe vitamin B12 deficiency in an infant associated with maternal deficiency and a strict vegetarian diet. J Ped Hem Oncol. 26:270-1
-
Jarosz et al, 2004 Vitamin B12 deficiency anaemia in a 7.5 months old girl. Med Wieku Rozwoj 8:283-8
-
Baatenburg et al 2006 Developmental delay in breastfed children due to inadequate diet of the mother. Ned Tijdshr Geneeskd 150: 465-9
-
Kollee 2006 Vitamin deficiencies in breastfed children due to maternal dietary deficiency. Ned Tijdschr Geneeskd. 150:473-6
-
Yajnik 2006 Nutritional control of fetal growth. Nutr Rev. 64: S50-1
-
Cetinkaya etal, 2007 Nutritional vitamin B12 deficiency in hospitalized young children. Ped. Hem. Onco. 24:15-21
-
Fadyl and Inoue 2007 Combined B12 and iron deficiency in a child breast-fed by a vegetarian mother. J Ped Hemato Oncol. 29:74
-
Mathey et al, 2007 Failure to thrive and psychomotor regression revealing vitamin B12 deficiency in 3 infants. Arch Ped 14: 467-71
-
Dror and Allen, 2008 Effect of vitamin B12 deficiency on neurodevelopment in infants... Nutr Rev. 66:250-5
-
Honzik et al, 2010 Clinical presentation and metabolic consequences in 40 breastfed infants... Eur J Paed Neurol. 14:488-95
-
Kocaoglu et al, 2014 Cerebral atrophy in a vitamin B12-deficient infant of a vegetarian mother. J Health Pop Nutr 32:367-71
-
Bousselamati et al. 2018 Psychomotor regression due to vitamin B12 deficiency. Pan Afr MEd J 20:30
-
Bravo etal, 2014 Haematological and neurological compromise due to vitamin B12 deficient in infant of a vegetarian mother.... PMID 25697251
-
Schroder etal 2017 Pregnant women of South Asian Ethnicity in Canada have substantially lower vitamin B12 status compared with pregnant woemen of European ethnicity PMID 28920568
-
Chandyo etal, 2017 The effects of vitamin B12 supplementation during pregnancy PMID 28851784
-
Woods etal, 1960 Vitamin B12Co-60 readily passes the placenta into fetal organs and nursing provides B12 from mother to pup... PMC 2137236
-
Graber etal, 1971 Placental transport of vitamin B12 in the pregnant rat PMID 5552402
-
Michelson etal, 1999 Urinary organic acid screening in children with developmental language delay
-
Specker et al. 1990 Vitamin B12: Low milk concentrations are related to low serum concentrations in vegetarian women.....Am J Clin Nutr 52:1073-6
-
Specker et al. 1994 Vegetarian diets during lactation. Am J. Clin Nutr 59:1182S-6S
-
Davis and Melina 2014 Becoming vegan: comprehensive addition.
-
Strand etal, 2015 Vitamin B12, folic acid, and growth in 6- to 30-month-old children... Pediatrics 135;918-26
-
Benbir etal, 2007 Seizures during treatment of vitamin B12 deficiency. Seizure. 2007 Jan;16(1):69-73. Epub 2006 Dec 5
-
Gao Q, Bi D, Li B, Ni M, Pang D, Li X, Zhang X, Xu Y, Zhao Q, Zhu C. The Association Between Branched-Chain Amino Acid Concentrations and the Risk of Autism Spectrum Disorder in Preschool-Aged Children. Mol Neurobiol. 2024 Aug;61(8):6031-6044. doi: 10.1007/s12035-024-03965-4. Epub 2024 Jan 24. PMID: 38265552; PMCID: PMC11249470.
-
Nowaczyk MJ, Lehotay DC, Platt BA, Fisher L, Tan R, Phillips H, Clarke JT. Ethylmalonic and methylsuccinic aciduria in ethylmalonic encephalopathy arise from abnormal isoleucine metabolism. Metabolism. 1998 Jul;47(7):836-9. doi: 10.1016/s0026-0495(98)90122-6. PMID: 9667231.
-
Kałużna-Czaplińska, J., Michalska, M., & Rynkowski, J. (2011). Homocysteine level in urine of autistic and healthy children.
-
Altun, H., Kurutaş, E. B., Şahin, N., Güngör, O., & Fındıklı, E. (2018). The Levels of Vitamin D, Vitamin D Receptor, Homocysteine and Complex B Vitamin in Children with Autism Spectrum Disorders.
Nitrous Oxide and Vitamin B12 deficiency
Vive MGD, Anguelova GV, Duim S, Hofstee HMA. Metabolic encephalopathy caused by nitrous oxide ('laughing gas') induced hyperammonaemia. BMJ Case Rep. 2019 Nov 25;12(11). pii: e232163. doi: 10.1136/bcr-2019-232163. PubMed PMID: 31772134.
Neveu J, Perelman S, Suisse G, Monpoux F. Severe hyperhomocysteinemia and peripheral neuropathy as side effects of nitrous oxide in two patients with
sickle cell disease. Arch Pediatr. 2019 Oct;26(7):419-421. doi: 10.1016/j.arcped.2019.09.006. Epub 2019 Oct 17. PubMed PMID: 31630905.
Edigin E, Ajiboye O, Nathani A. Nitrous Oxide-induced B12 Deficiency Presenting With Myeloneuropathy. Cureus. 2019 Aug 6;11(8):e5331. doi:10.7759/cureus.5331. PubMed PMID: 31598438; PubMed Central PMCID: PMC6777927.
Tani J, Weng HY, Chen HJ, Chang TS, Sung JY, Lin CS. Elucidating Unique Axonal Dysfunction Between Nitrous Oxide Abuse and Vitamin B12 Deficiency. Front Neurol. 2019 Jul 9;10:704. doi: 10.3389/fneur.2019.00704. eCollection 2019. PubMed PMID: 31354607; PubMed Central PMCID: PMC6633399.
Nouri A, Patel K, Montejo J, Nasser R, Gimbel DA, Sciubba DM, Cheng JS. The Role of Vitamin B(12) in the Management and Optimization of Treatment in Patients With Degenerative Cervical Myelopathy. Global Spine J. 2019 May;9(3):331-337. doi: 10.1177/2192568218758633. Epub 2018 May 17. Review. PubMed PMID: 31192102; PubMed Central PMCID: PMC6542160.
Gullestrup A, Jensen RB, Břgevig S, Nilsson PM. [Acute neuropathy and liver injury following the abuse of nitrous oxide]. Ugeskr Laeger. 2019 May 13;181(20).
pii: V12180890. Danish. PubMed PMID: 31124452.
Norris F, Mallia P. Lesson of the month 2: A case of nitrous oxide-induced pancytopenia. Clin Med (Lond). 2019 Mar;19(2):129-130. doi: 10.7861/clinmedicine.19-2-129. PubMed PMID: 30872294; PubMed Central PMCID:
PMC6454366.
Williamson J, Huda S, Damodaran D. Nitrous oxide myelopathy with functional vitamin B (12) deficiency. BMJ Case Rep. 2019 Feb 13;12(2). pii: e227439. doi:
10.1136/bcr-2018-227439. PubMed PMID: 30765444.
Lundin MS, Cherian J, Andrew MN, Tikaria R. One month of nitrous oxide abuse causing acute vitamin B (12) deficiency with severe neuropsychiatric symptoms.
BMJ Case Rep. 2019 Feb 7;12(2). pii: bcr-2018-228001. doi: 10.1136/bcr-2018-228001. PubMed PMID: 30737329.
Lan SY, Kuo CY, Chou CC, Kong SS, Hung PC, Tsai HY, Chen YC, Lin JJ, Chou IJ, Lin KL; PCHAN Study Group. Recreational nitrous oxide abuse related subacute combined degeneration of the spinal cord in adolescents - A case series and literature review. Brain Dev. 2019 May;41(5):428-435. doi: 10.1016/j.braindev.2018.12.003. Epub 2019 Jan 2. Review. PubMed PMID: 30611595.
Dong X, Ba F, Wang R, Zheng D. Imaging appearance of myelopathy secondary to nitrous oxide abuse: a case report and review of the literature. Int J Neurosci.
2019 Mar;129(3):225-229. doi: 10.1080/00207454.2018.1526801. Epub 2018 Dec 4.Review. PubMed PMID: 30234413.
Patel KK, Mejia Munne JC, Gunness VRN, Hersey D, Alshafai N, Sciubba D, Nasser R, Gimbel D, Cheng J, Nouri A. Subacute combined degeneration of the
spinal cord following nitrous oxide anesthesia: A systematic review of cases. Clin Neurol Neurosurg. 2018 Oct;173:163-168. doi: 10.1016/j.clineuro.2018.08.016.
Epub 2018 Aug 9. Erratum in: Clin Neurol Neurosurg. 2019 Feb;177:123-124. Abstract corrected. PubMed PMID: 30144777.
Jolobe OMP. Other aspects of nitrous oxide-related neuromyelopathy. Am J Emerg Med. 2019 Feb;37(2):350-351. doi: 10.1016/j.ajem.2018.05.076. Epub 2018 May 30. PubMed PMID: 29866413.
Egan W, Steinberg E, Rose J. Vitamin B(12) deficiency-induced neuropathy secondary to prolonged recreational use of nitrous oxide. Am J Emerg Med. 2018
Sep;36(9):1717.e1-1717.e2. doi: 10.1016/j.ajem.2018.05.029. Epub 2018 May 24. PubMed PMID: 29859645.
Anderson D, Beecher G, van Dijk R, Hussain M, Siddiqi Z, Ba F. Subacute Combined Degeneration from Nitrous Oxide Abuse in a Patient with Pernicious
Anemia. Can J Neurol Sci. 2018 May;45(3):334-335. doi: 10.1017/cjn.2018.15. PubMed PMID: 29756593.
Antonucci MU. Subacute Combined Degeneration from Recreational Nitrous Oxide Inhalation. J Emerg Med. 2018 May;54(5):e105-e107. doi: 10.1016/j.jemermed.2018.01.045. Epub 2018 Mar 27. PubMed PMID: 29602528.
Keddie S, Adams A, Kelso ARC, Turner B, Schmierer K, Gnanapavan S, Malaspina A, Giovannoni G, Basnett I, Noyce AJ. No laughing matter: subacute degeneration of the spinal cord due to nitrous oxide inhalation. J Neurol. 2018 May;265(5):1089-1095. doi: 10.1007/s00415-018-8801-3. Epub 2018 Mar 3. PubMed
PMID: 29502317; PubMed Central PMCID: PMC5937900.
Johnson K, Mikhail P, Kim MG, Bosco A, Huynh W. Recreational nitrous oxide-associated neurotoxicity. J Neurol Neurosurg Psychiatry. 2018 Aug;89(8):897-898. doi: 10.1136/jnnp-2017-317768. Epub 2018 Jan 24. PubMed PMID: 29367261.
Al-Sadawi M, Claris H, Archie C, Jayarangaiah A, Oluya M, McFarlane SI. Inhaled Nitrous Oxide 'Whip-Its!' Causing Subacute Combined Degeneration of
Spinal Cord. Am J Med Case Rep. 2018;6(12):237-240. doi: 10.12691/ajmcr-6-12-3. Epub 2018 Dec 26. PubMed PMID: 31058215; PubMed Central PMCID: PMC6499494.
Friedlander G, Davies T. The Last Laugh - Reversible myeloneuropathy induced by chronic nitrous oxide use. Acute Med. 2018;17(4):232-235. PubMed PMID:
30882108.
Yuan JL, Wang SK, Jiang T, Hu WL. Nitrous oxide induced subacute combined degeneration with longitudinally extensive myelopathy with inverted V-sign on
spinal MRI: a case report and literature review. BMC Neurol. 2017 Dec 28;17(1):222. doi: 10.1186/s12883-017-0990-3. PubMed PMID: 29282001; PubMed
Central PMCID: PMC5745895.
Conjaerts SHP, Bruijnes JE, Beerhorst K, Beekman R. [Nitrous oxide-induced polyneuropathy]. Ned Tijdschr Geneeskd. 2017;161:D2044. Dutch. PubMed PMID:
29192578.
Kaski D, Kumar P, Murphy E, Warner TT. Iatrogenic B12-deficient peripheral neuropathy following nitrous oxide administration for functional tonic leg spasm:
A case report. Clin Neurol Neurosurg. 2017 Sep;160:108-110. doi:10.1016/j.clineuro.2017.07.006. Epub 2017 Jul 6. PubMed PMID: 28709008.
Stockton L, Simonsen C, Seago S. Nitrous oxide-induced vitamin B12 deficiency. Proc (Bayl Univ Med Cent). 2017 Apr;30(2):171-172. PubMed PMID: 28405070; PubMed Central PMCID: PMC5349816.
Buizert A, Sharma R, Koppen H. When the Laughing Stops: Subacute Combined Spinal Cord Degeneration Caused by Laughing Gas Use. J Addict Med. 2017
May/Jun;11(3):235-236. doi: 10.1097/ADM.0000000000000295. PubMed PMID: 28166085.
40: Chen HJ, Huang CS. Nitrous Oxide-induced Subacute Combined Degeneration Presenting with Dystonia and Pseudoathetosis: A Case Report. Acta Neurol Taiwan. 2016 Jun 15;25(2):50-55. PubMed PMID: 27854092.
Mancke F, Kaklauskaitė G, Kollmer J, Weiler M. Psychiatric comorbidities in a young man with subacute myelopathy induced by abusive nitrous oxide consumption: a case report. Subst Abuse Rehabil. 2016 Sep 29;7:155-159. eCollection 2016.PubMed PMID: 27729826; PubMed Central PMCID: PMC5047713.
Massey TH, Pickersgill TT, J Peall K. Nitrous oxide misuse and vitamin B12 deficiency. BMJ Case Rep. 2016 May 31;2016. pii: bcr2016215728. doi:10.1136/bcr-2016-215728. PubMed PMID: 27247211; PubMed Central PMCID: PMC4904416.
Duque MA, Kresak JL, Falchook A, Harris NS. Nitrous Oxide Abuse and Vitamin B12 Action in a 20-Year-Old Woman: A Case Report. Lab Med. 2015 Fall;46(4):312-5. doi: 10.1309/LM0L9HAVXCHF1UQM. PubMed PMID: 26489675.
Pugliese RS, Slagle EJ, Oettinger GR, Neuburger KJ, Ambrose TM. Subacute combined degeneration of the spinal cord in a patient abusing nitrous oxide and
self-medicating with cyanocobalamin. Am J Health Syst Pharm. 2015 Jun 1;72(11):952-7. doi: 10.2146/ajhp140583. PubMed PMID: 25987690.
Morris N, Lynch K, Greenberg SA. Severe motor neuropathy or neuronopathy due to nitrous oxide toxicity after correction of vitamin B12 deficiency. Muscle
Nerve. 2015 Apr;51(4):614-6. doi: 10.1002/mus.24482. Epub 2015 Feb 24. PubMedPMID: 25297001.
Garakani A, Welch AK, Jaffe RJ, Protin CA, McDowell DM. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics.
2014 Nov-Dec;55(6):715-9. doi: 10.1016/j.psym.2013.11.001. Epub 2013 Nov 5.PubMed PMID: 24367897.
Safari A, Emadi F, Jamali E, Borhani-Haghighi A. Clinical and MRI manifestations of nitrous oxide induced vitamin B12 deficiency: A case report. Iran J Neurol. 2013;12(3):111-3. PubMed PMID: 24250916; PubMed Central PMCID: PMC3829298.
Chiang TT, Hung CT, Wang WM, Lee JT, Yang FC. Recreational nitrous oxide abuse-induced vitamin B12 deficiency in a patient presenting with
hyperpigmentation of the skin. Case Rep Dermatol. 2013 Jun 29;5(2):186-91. doi: 10.1159/000353623. Print 2013 May. PubMed PMID: 23898268; PubMed Central PMCID: PMC3724136.
Chaugny C, Simon J, Collin-Masson H, De Beauchęne M, Cabral D, Fagniez O, Veyssier-Belot C. [Vitamin B12 deficiency due to nitrous oxide use: unrecognized
cause of combined spinal cord degeneration]. Rev Med Interne. 2014 May;35(5):328-32. doi: 10.1016/j.revmed.2013.04.018. Epub 2013 Jun 14. French. PubMed PMID: 23773901.
Cheng HM, Park JH, Hernstadt D. Subacute combined degeneration of the spinal cord following recreational nitrous oxide use. BMJ Case Rep. 2013 Mar 8;2013.
pii: bcr2012008509. doi: 10.1136/bcr-2012-008509. PubMed PMID: 23476009; PubMed Central PMCID: PMC3618752.
Ghobrial GM, Dalyai R, Flanders AE, Harrop J. Nitrous oxide myelopathy posing as spinal cord injury. J Neurosurg Spine. 2012 May;16(5):489-91. doi:10.3171/2012.2.SPINE11532. Epub 2012 Mar 2. PubMed PMID: 22385084.
Probasco JC, Felling RJ, Carson JT, Dorsey ER, Niessen TM. Teaching NeuroImages: myelopathy due to B₁₂ deficiency in long-term colchicine treatment
and nitrous oxide misuse. Neurology. 2011 Aug 30;77(9):e51. doi:10.1212/WNL.0b013e31822c910f. PubMed PMID: 21876193.
Lin RJ, Chen HF, Chang YC, Su JJ. Subacute combined degeneration caused by nitrous oxide intoxication: case reports. Acta Neurol Taiwan. 2011 Jun;20(2):129-37. Review. PubMed PMID: 21739392.
Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: Perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011 Feb
15;301(1-2):1-8. doi: 10.1016/j.jns.2010.10.033. Epub 2010 Nov 26. Review. PubMed PMID: 21112598.
Alt RS, Morrissey RP, Gang MA, Hoffman RS, Schaumburg HH. Severe myeloneuropathy from acute high-dose nitrous oxide (N2O) abuse. J Emerg Med. 2011
Oct;41(4):378-80. doi: 10.1016/j.jemermed.2010.04.020. Epub 2010 Jun 7. PubMed PMID: 20605391.
Richardson PG. Peripheral neuropathy following nitrous oxide abuse. Emerg Med Australas. 2010 Feb;22(1):88-90. doi: 10.1111/j.1742-6723.2009.01262.x. PubMedPMID: 20152009.
Wijesekera NT, Davagnanam I, Miszkiel K. Subacute combined cord degeneration: a rare complication of nitrous oxide misuse. A case report. Neuroradiol J. 2009
May 15;22(2):194-7. Epub 2009 May 15. PubMed PMID: 24207040.
Renard D, Dutray A, Remy A, Castelnovo G, Labauge P. Subacute combined degeneration of the spinal cord caused by nitrous oxide anaesthesia. Neurol Sci.
2009 Feb;30(1):75-6. doi: 10.1007/s10072-009-0013-2. Epub 2009 Jan 24. PubMed PMID: 19169627.
Jameson M, Roberts S, Anderson NE, Thompson P. Nitrous oxide-induced vitamin B(12) deficiency. J Clin Neurosci. 1999 Mar;6(2):164-6. PubMed PMID: 18639144.
Sethi NK, Mullin P, Torgovnick J, Capasso G. Nitrous oxide "whippit" abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006 Jun;2(2):71-4. Review. PubMed PMID: 18072118; PubMed Central PMCID: PMC3550053.
Krajewski W, Kucharska M, Pilacik B, Fobker M, Stetkiewicz J, Nofer JR, Wronska-Nofer T. Impaired vitamin B12 metabolic status in healthcare workers
occupationally exposed to nitrous oxide. Br J Anaesth. 2007 Dec;99(6):812-8. Epub 2007 Oct 20. PubMed PMID: 17951609.
Wu MS, Hsu YD, Lin JC, Chen SC, Lee JT. Spinal myoclonus in subacute combined degeneration caused by nitrous oxide intoxication. Acta Neurol Taiwan. 2007
Jun;16(2):102-5. PubMed PMID: 17685135.
Singer MA, Lazaridis C, Nations SP, Wolfe GI. Reversible nitrous oxide-induced myeloneuropathy with pernicious anemia: case report and literature review. Muscle Nerve. 2008 Jan;37(1):125-9. PubMed PMID: 17623854.
Cohen Aubart F, Sedel F, Vicart S, Lyon-Caen O, Fontaine B. [Nitric-oxide triggered neurological disorders in subjects with vitamin B12 deficiency]. Rev Neurol (Paris). 2007 Mar;163(3):362-4. French. PubMed PMID: 17404524.
Ahn SC, Brown AW. Cobalamin deficiency and subacute combined degeneration after nitrous oxide anesthesia: a case report. Arch Phys Med Rehabil. 2005 Jan;86(1):150-3. PubMed PMID: 15641006.
Miller MA, Martinez V, McCarthy R, Patel MM. Nitrous oxide "whippit" abuse presenting as clinical B12 deficiency and ataxia. Am J Emerg Med. 2004 Mar;22(2):124. PubMed PMID: 15011232.
Waclawik AJ, Luzzio CC, Juhasz-Pocsine K, Hamilton V. Myeloneuropathy from nitrous oxide abuse: unusually high methylmalonic acid and homocysteine levels.
WMJ. 2003;102(4):43-5. Erratum in: WMJ. 2003;102(6):5. PubMed PMID: 12967021.
Ilniczky S, Jelencsik I, Kenéz J, Szirmai I. MR findings in subacute combined degeneration of the spinal cord caused by nitrous oxide anaesthesia--two cases.
Eur J Neurol. 2002 Jan;9(1):101-4. PubMed PMID: 11784385.
Barbosa L, Leal I, Timóteo AT, Matias T. [Acute megaloblastic anemia caused by inhalation of nitrous oxide in a patient with multiple autoimmune pathology]. Acta Med Port. 2000 Sep-Dec;13(5-6):309-12. Portuguese. PubMed PMID: 11234497.
Deleu D, Hanssens Y, Louon A. Nitrous oxide-induced cobalamin deficiency. Arch Neurol. 2001 Jan;58(1):134-5. PubMed PMID: 11176951.
McNeely JK, Buczulinski B, Rosner DR. Severe neurological impairment in an infant after nitrous oxide anesthesia. Anesthesiology. 2000 Dec;93(6):1549-50.
PubMed PMID: 11149458.
Felmet K, Robins B, Tilford D, Hayflick SJ. Acute neurologic decompensation in an infant with cobalamin deficiency exposed to nitrous oxide. J Pediatr. 2000 Sep;137(3):427-8. PubMed PMID: 10969273.
Marié RM, Le Biez E, Busson P, Schaeffer S, Boiteau L, Dupuy B, Viader F. Nitrous oxide anesthesia-associated myelopathy. Arch Neurol. 2000 Mar;57(3):380-2. PubMed PMID: 10714665.
Göthe CJ, Petersson G. [Nitrous oxide and cobalamin deficiency]. Lakartidningen. 1999 Dec 15;96(50):5609. Swedish. PubMed PMID: 10643221.
Lindstedt G. [Nitrous oxide can cause cobalamin deficiency. Vitamin B12 is a simple and cheap remedy]. Lakartidningen. 1999 Nov 3;96(44):4801-5. Review.
Swedish. PubMed PMID: 10584542.
Alarcia R, Ara JR, Serrano M, García M, Latorre AM, Capablo JL. [Severe polyneuropathy after using nitrous oxide as an anesthetic. A preventable disease?]. Rev Neurol. 1999 Jul 1-15;29(1):36-8. Spanish. PubMed PMID: 10528308.
Sesso RM, Iunes Y, Melo AC. Myeloneuropathy following nitrous oxide anesthaesia in a patient with macrocytic anaemia. Neuroradiology. 1999 Aug;41(8):588-90. PubMed PMID: 10447571.
Mayall M. Vitamin B12 deficiency and nitrous oxide. Lancet. 1999 May 1;353(9163):1529. PubMed PMID: 10232347.
Pema PJ, Horak HA, Wyatt RH. Myelopathy caused by nitrous oxide toxicity. AJNR Am J Neuroradiol. 1998 May;19(5):894-6. PubMed PMID: 9613506.
Beltramello A, Puppini G, Cerini R, El-Dalati G, Manfredi M, Roncolato G, Idone D, De Togni L, Turazzini M. Subacute combined degeneration of the spinal
cord after nitrous oxide anaesthesia: role of magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 1998 Apr;64(4):563-4. PubMed PMID: 9576560; PubMed
Central PMCID: PMC2170040.
Horne DW, Holloway RS. Compartmentation of folate metabolism in rat pancreas: nitrous oxide inactivation of methionine synthase leads to accumulation of 5-methyltetrahydrofolate in cytosol. J Nutr. 1997 Sep;127(9):1772-5. PubMed PMID: 9278558.
Takács J. [N2O-induced acute funicular myelosis in latent vitamin B 12 deficiency]. Anasthesiol Intensivmed Notfallmed Schmerzther. 1996 Oct;31(8):525-8. German. PubMed PMID: 9019188.
Nestor PJ, Stark RJ. Vitamin B12 myeloneuropathy precipitated by nitrous oxide anaesthesia. Med J Aust. 1996 Aug 5;165(3):174. PubMed PMID: 8709889.
Rösener M, Dichgans J. Severe combined degeneration of the spinal cord after nitrous oxide anaesthesia in a vegetarian. J Neurol Neurosurg Psychiatry. 1996
Mar;60(3):354. PubMed PMID: 8609528; PubMed Central PMCID: PMC1073874.
Hadzic A, Glab K, Sanborn KV, Thys DM. Severe neurologic deficit after nitrous oxide anesthesia. Anesthesiology. 1995 Oct;83(4):863-6. Review. PubMed PMID: 7574068.
King M, Coulter C, Boyle RS, Whitby RM. Neurotoxicity from overuse of nitrous oxide. Med J Aust. 1995 Jul 3;163(1):50-1. PubMed PMID: 7609693.
Young PB, Kennedy S, Molloy AM, Scott JM, Weir DG, Kennedy DG. Effect of N2O treatment/vitamin B12 deficiency in pigs on tissue concentrations of odd-numbered, branched-chain fatty acids. Int J Vitam Nutr Res.1995;65(4):255-60. PubMed PMID: 8789622.
Louis-Ferdinand RT. Myelotoxic, neurotoxic and reproductive adverse effects of nitrous oxide. Adverse Drug React Toxicol Rev. 1994 Winter;13(4):193-206.Review. PubMed PMID: 7734639.
Flippo TS, Holder WD Jr. Neurologic degeneration associated with nitrous oxide anesthesia in patients with vitamin B12 deficiency. Arch Surg. 1993 Dec;128(12):1391-5. Review. PubMed PMID: 8250714.
Carmel R, Rabinowitz AP, Mazumder A. Metabolic evidence of cobalamin deficiency in bone marrow cells harvested for transplantation from donors given nitrous oxide. Eur J Haematol. 1993 Apr;50(4):228-33. PubMed PMID: 8500605.
Koblin DD, Tomerson BW, Waldman FM, Lampe GH, Wauk LZ, Eger EI 2nd. Effect of nitrous oxide on folate and vitamin B12 metabolism in patients. Anesth Analg. 1990 Dec;71(6):610-7. PubMed PMID: 2240633.
Koblin DD, Tomerson BW, Waldman FM. Disruption of folate and vitamin B12 metabolism in aged rats following exposure to nitrous oxide. Anesthesiology. 1990 Sep;73(3):506-12. PubMed PMID: 2393136.
van Achterbergh SM, Vorster BJ, Heyns AD. The effect of sepsis and short-term exposure to nitrous oxide on the bone marrow and the metabolism of vitamin B12 and folate. S Afr Med J. 1990 Sep 1;78(5):260-3. PubMed PMID: 2392722.
van der Westhuyzen J, Davis RE, Icke GC, Metz J. Tissue folates in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency and neurological impairment. Br J Nutr. 1987 Nov;58(3):485-91. PubMed PMID:3120768.
Van de List C, Combs M, Schilling RF. Nitrous oxide and vitamin B12 deficiency interact adversely on rat growth. J Lab Clin Med. 1986 Oct;108(4):346-8. PubMed PMID: 3760674.
Koblin DD, Biebuyck JF. Is nitrous oxide a dangerous anesthetic for vitamin B12-deficient subjects? JAMA. 1986 Aug 8;256(6):716. PubMed PMID: 3723770.
Schilling RF. Is nitrous oxide a dangerous anesthetic for vitamin B12-deficient subjects? JAMA. 1986 Mar 28;255(12):1605-6. PubMed PMID: 3951096.
McLoughlin JL, Cantrill RC. Nitrous oxide induced vitamin B12 deficiency: measurement of methylation reactions in the fruit bat (Rousettus aegyptiacus).
Int J Biochem. 1986;18(2):199-202. PubMed PMID: 3949064.
Wilson SD, Horne DW. Effect of nitrous oxide inactivation of vitamin B12 on the levels of folate coenzymes in rat bone marrow, kidney, brain, and liver. Arch Biochem Biophys. 1986 Jan;244(1):248-53. PubMed PMID: 3947060.
van Tonder SV, Ruck A, van der Westhuyzen J, Fernandes-Costa F, Metz J. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of
DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986 Jan;55(1):187-92. PubMed PMID: 3663573.
van der Westhuyzen J, van Tonder SV, Gibson JE, Kilroe-Smith TA, Metz J.Plasma amino acids and tissue methionine levels in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1985 May;53(3):657-62. PubMed PMID: 4063293.
O'Leary PW, Combs MJ, Schilling RF. Synergistic deleterious effects of nitrous oxide exposure and vitamin B12 deficiency. J Lab Clin Med. 1985 Apr;105(4):428-31. PubMed PMID: 3981056.
van der Westhuyzen J, Metz J. Betaine delays the onset of neurological impairment in nitrous oxide-induced vitamin B-12 deficiency in fruit bats. J Nutr. 1984 Jun;114(6):1106-11. PubMed PMID: 6726473.
van der Westhuyzen J, Fernandes-Costa F, Metz J. Cobalamin inactivation by nitrous oxide produces severe neurological impairment in fruit bats : protection by methionine and aggravation by folates. Life Sci. 1982 Nov 1;31(18):2001-10. PubMed PMID: 7176808.
Lumb M, Perry J, Deacon R, Chanarin I. Urinary folate loss following inactivation of vitamin B12 by nitrous oxide in rats. Br J Haematol. 1982 Jun;51(2):235-42. PubMed PMID: 7082582.
Kondo H, Osborne ML, Kolhouse JF, Binder MJ, Podell ER, Utley CS, Abrams RS, Allen RH. Nitrous oxide has multiple deleterious effects on cobalamin metabolism and causes decreases in activities of both mammalian cobalamin-dependent enzymes in rats. J Clin Invest. 1981 May;67(5):1270-83. PubMed PMID: 6112240; PubMed Central PMCID: PMC370693.
Steinberg SE, Campbell C, Hillman RS. The effect of nitrous oxide-induced vitamin B12 deficiency on in vivo folate metabolism. Biochem Biophys Res Commun.
1981 Feb 27;98(4):983-9. PubMed PMID: 6164371.
McKenna B, Weir DG, Scott JM. The induction of functional vitamin B-12 deficiency in rats by exposure to nitrous oxide. Biochim Biophys Acta. 1980 Mar 20;628(3):314-21. PubMed PMID: 7370297.
Lumb M, Deacon R, Perry J, Chanarin I, Minty B, Halsey MJ, Nunn JF. The effect of nitrous oxide inactivation of vitamin B12 on rat hepatic folate.Implications for the methylfolate-trap hypothesis. Biochem J. 1980 Mar 15;186(3):933-6. PubMed PMID: 7396845; PubMed Central PMCID: PMC1161731.
Adornato BT. Nitrous oxide and vitamin B12. Lancet. 1978 Dec 16;2(8103):1318. PubMed PMID: 82831.
Deacon R, Lumb M, Perry J, Chanarin I, Minty B, Halsey MJ, Nunn JF. Selective inactivation of vitamin B12 in rats by nitrous oxide. Lancet. 1978 Nov
11;2(8098):1023-4. PubMed PMID: 82036.
Amess JA, Burman JF, Rees GM, Nancekievill DG, Mollin DL. Megaloblastic haemopoiesis in patients receiving nitrous oxide. Lancet. 1978 Aug12;2(8085):339-42. PubMed PMID: 79709.Flodh H, Ullberg S. Accumulation of labelled vitamin B12 in some transplanted tumours. Int J Cancer. 1968 Sep 15;3(5):694-9. doi: 10.1002/ijc.2910030518. PMID: 5724527. ANW,
Workinger JL, Nexo E, Doyle RP, Viola-Villegas N. 89Zr-Cobalamin PET Tracer: Synthesis, Cellular Uptake, and Use for Tumor Imaging. ACS Omega. 2017 Oct 31;2(10):6314-6320. doi: 10.1021/acsomega.7b01180. Epub 2017 Oct 2. PMID: 29104950; PMCID: PMC5664145.
Copyright © 2014 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written permission is prohibited