There are over 200 enzymes in the body involved in methylation
A critical methylation reaction is the formation of Melatonin.
Melatonin is critical for sleep
Functional B12 deficiency results in sleep disorders
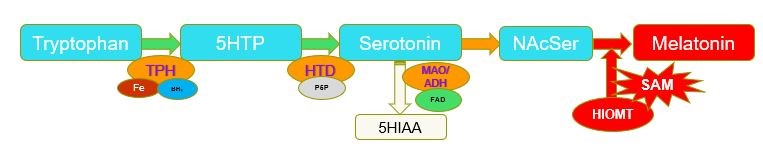
Synthesis of Melatonin through methylation of N-ActylSerotonin by the enzyme Hydroxyindole-O-methyl Transferase (HIOMT). N-AcetylSerotonin-[HIOMT] + SAM => Melatonin=[HIOMT] + SAH (Gallardo and Tamezzani 1975; Klein and Lines, 1969; Urry etal, 1972; Quay 1965;Kuwano and Takahashi, 1980; Yokim and Wallen 1975; ). Deficiency in methyl B12, results in reduced levels of SAM, which in turn leads to conditions such as poor sleep, poor maturation of the gut wall, and developmental delay due to lack of activation of neuronal stem cells and subsequent differentiation into myelin-producing oligodendrocytes in the brain.
Melatonin and analogs that bind to the melatonin receptors are important because of their role in the management of depression, insomnia, epilepsy, Alzheimer’s disease (AD), diabetes, obesity, alopecia, migraine, cancer, and immune and cardiac disorders.
It is known that melatonin receptors are expressed in the fetal brain. During pregnancy melatonin levels rise in the mother, and is transferred transplacentally to the fetus. In disorders of pregnancy, melatonin levels are decreased in both the mother and fetus. Further it has also been found that alterations in the levels of maternal melatonin have been associated with disrupted brain programming and long-term sequelae (Sagrillo-Fagundes etal, 2016). The newborn also does not produce its own melatonin and is thus dependent upon melatonin supplied to the baby via milk. The neonate has not developed the pineal gland and maturation of pineal function is required before rhythmic melatonin production is achieved in the new-born (roughly 9-12 weeks after birth). The diurnal production of melatonin by the mother will therefore dictate the melatonin levels in milk. Late in fetal development the fetuses’ sleep patterns develop through the regulation of melatonin.
Reduced levels of B12 in pregnant mothers would lead to lower methylation and hence lower production of melatonin, both in the mothers, and then in the foetus/new-born. Reduced methylation has been shown to occur in ASD kids (James etal, 2014).
Reduced levels of melatonin in the brain of ASD would also affect sleeping patterns in these children, and sleep disorders are a common feature of ASD, with around 80% of children “suffering” from the condition( Blackmer and Feinstein 2016; Kotagal and Broomall, 2012).
In Methyl B12 deficiency, there is a greatly reduced production of SAM, and in an attempt to overcome the deficiency the cell tries to accommodate by producing more and more precursors for the production of melatonin and adrenalin. However in severe deficiency this then leads to a significant increase in the breakdown products of dopamine and nor-adrenalin (HVA and VMA) and breakdown products of tryptophan metabolis, Kynurenic acid (KA) and Quinolinic acid (QA), As well there is an increase in the breakdown product of Serotonin, 5-Hydroxyindoleacetic acid (5HIAA) start to accumulate and can be detected as elevated levels in urine.
A deficiency in functional Methyl B12 leads to reduced production of melatonin, thereby increasing the incidence of sleep disorders. Sleep disorders are very common in those with functional B12 deficiency and are particularly prevalent in conditions associated with functional vitamin B12 deficiency, such as dementia (Benca and Teodorescu., 2019; Cipriani etal, 2015; Shenker and Sing, 2017), autism and CFS. Despite the obvious role of methylation in the formation of melatonin, and its role in promotion of sleep, few researchers seem to understand this. Insomnia, or difficulty sleeping is common in neurological development disorders such as Autism 53-80%, Ballester etal, 2020; Kohyama 2016; Blackmer and Feinstein, 2016; Robinson-Shelton and Malow, 2016; Geier etal, 2012; Gringras et al 2017; Maras et al, 2018; Devnani and Hedge, 2015; Cagnon and Godbout, 2018; Gobi and Comai, 2019; Esposito etal, 2019). A new diagnostic Pediatric Sleep Clinical Global Impressions Scale has recently been developed as an aid to diagnosis (Malow et al, 2016). Sleep disorders occur in only 1-2% of children with normal development but in 80% of kids with developmental disorders such as Autism. Often such disorders continue through to early childhood and can lead to behavioural problems at school Hirata etal, 2016). Problems are encountered with sleep duration, night wakenings and bedtime resistance. Difficulty in sleeping is likely to be due to the lower levels of melatonin produced in ASD kids. Many doctors though, treat sleep disorders with melatonin, or delayed release forms of melatonin, rather than fixing the functional B12 deficiency (Quera-Salva and Claustrat, 2018; Esposito etal, 2019; Huysmans etal, 2019). More specifically institutes such as the Sleep Health Foundation seem to have no idea about the connection.
A recent study looking at markers of methyl vitamin B12 in 10 randomly selected children under 2 years old, who had continued sleep deprivation revealed an anomalously high markers of functional vitamin B12 deficiency (Russell-Jones, 2022). Representative data is presented below. These individuals also had metabolic signs of functional vitamin B2 deficiency, suggesting that the observed functional B12 deficiency was linked to reduced levels of functional vitamin B2. Hence, there were increased levels of glutaric acid, adipic acid and suberic acid.
As can be seen there are highly elevated levels of HVA/VMA/QA/KA/Adipic Acid, Ethylmalonate, Succinate and Suberic Acid, in the 10 individuals with sleep disorder, in comparison to the Normal situation. The data strongly supports the concept that functional B2 deficiency results in functional B12 deficiency. The functional B12 deficiency, then results in decreased melatonin production and an extreme elevation of the neurotransmiiter metabolites, HVA/VMA/QA/KA. Resolution of the sleep disorder has been found to require establishment of functional B2 sufficiency and treatment with repeated high dose Adenosyl/Methyl B12.
Restoration of brain vitamin B12 is quite complicated. There is no evidence that this can be done orally, and there are many studies that have been unsuccessful at low doses. Hence, daily oral administration of 2 ug Cyanocobalamin, was ineffective in restoring sleep patterns ( Hysing etal 2022). High dose oral B12 given ()1.5 mg 2 x bd was effective for restoration of sleep patterns during duration of treatment (Okawa, 1990), and 3 mg Methyl B12 per os per day (Ohta etal, 1991; Takahashi, etal, 1999). In paradoxical B12 deficiency, administration of cyanocobalamin is totally ineffective,, as FAD is required for reduction of the cyanide group, whilst adminstration of methyl B12 is only effective once the functional B2 deficiency is resolved (Okawa etal, 1999).
Successful treatment of sleep disorders in children has been achieved with the RnB protocol, in which functional vitamin B2 deficiency is resolved and is combined with topical sdministration of the B12 oils Adenosyl/Methyl B12 oils.
de Gallardo MR, Tramezzani JH Hydroxyindole-O-methyl-transferase activity in the pineal gland of the rabbit. .J Neural Transm. 1975;36(1):51-7. doi:
Klein DC, Lines SV. Pineal hydroxyindole-O-methyl transferase activity in the growing rat. Endocrinology. 1969 Jun;84(6):1523-5. doi: 10.1210/endo-84-6-1523.PMID: 5781132.
Urry RL, Barfuss DW, Ellis LC. Hydroxyindole-O-methyl transferase activity of male rat pineal glands following hypophysectomy and HGG treatment. Biol Reprod. 1972 Apr;6(2):238-43. doi: 10.1093/biolreprod/6.2.238.PMID: 5016871
Quay WB. Retinal and pineal hydroxyindole-o-methyl transferase activity in vertebrates. Life Sci. 1965 May;4(9):983-91. doi: 10.1016/0024-3205(65)90202-x.PMID: 5840097.
Kuwano R, Takahashi Y. S-adenosylhomocysteine is bound to pineal hydroxyindole O-methyl transferase. Life Sci. 1980 Oct 6;27(14):1321-6. doi: 10.1016/0024-3205(80)90226-x.PMID: 7003279
Yochim JM, Wallen EP. Correlation between hydroxyindole-O-methyl transferase rhythmicity and reproductive function in the rat. Adv Exp Med Biol. 1975;54:85-92. doi: 10.1007/978-1-4684-8715-2_4.PMID: 1168405
Ballester etal Sleep in autism: A biomolecular approach to aetiology and treatment. Sleep Med Rev. 2020 54:101357
Benca, RM and Teodorescu, M. Sleep physiology and disorders in aging and dementia. Handb Clin Neurol, 2019 167:477-493.
Cipriani etal. Sleep disturbances and dementia. Psychogeriatrics 2015 Mar;15(1):65-74
Shenker JI, and Singh G Sleep and Dementia Mo Med Jul-Aug 2017;114(4):311-315
Gringras P, Nir T, Breddy J, Frydman-Marom A, Findling RL Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children With Autism Spectrum Disorder. .J Am Acad Child Adolesc Psychiatry. 2017 Nov;56(11):948-957.e4. doi: 10.1016/j.jaac.2017.09.414. Epub 2017 Sep 19.
Athanasios Maras 1, Carmen M Schroder 2 3, Beth A Malow 4, Robert L Findling 5, John Breddy 6, Tali Nir 7, Shiri Shahmoon 7, Nava Zisapel 7, Paul Gringras 8 Long-Term Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder J Child Adolesc Psychopharmacol . 2018 Dec;28(10):699-710.
Preeti A Devnani 1, Anaita U Hegde 2 Autism and sleep disorders J Pediatr Neurosci Oct-Dec 2015;10(4):304-7. doi: 10.4103/1817-1745.174438.
Katia Gagnon 1 2, Roger Godbout 1 2 Melatonin and Comorbidities in Children with Autism Curr Dev Disord Rep 2018;5(3):197-206.
Gabriella Gobbi 1, Stefano Comai 1 2 Sleep well. Untangling the role of melatonin MT1 and MT2 receptors in sleep J Pineal Res 2019 Apr;66(3):e12544. doi: 10.1111/jpi.12544. Epub 2019 Jan 21
Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.;Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The serotonin-N-acetylserotonin–melatonin pathway as a biomarker for autism spectrum disorders. Transl. Psychiatry 2014, 4, e479
Miller SL, Yawno T, Alers NO, Castillo-Melendez M, Supramaniam VG, VanZyl N, Sabaretnam T, Loose JM, Drummond GR, Walker DW, Jenkin G, Wallace EM. Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J Pineal Res. 2014 Apr;56(3):283-94. doi: 10.1111/jpi.12121. Epub 2014 Feb 22. PMID: 24456220.
Susanna Esposito1, Daniela Laino2, Renato D'Alonzo2, Annalisa Mencarelli2, Lorenza Di Genova2, Antonella Fattorusso2, Alberto Argentiero3, Elisabetta Mencaroni2 Pediatric sleep disturbances and treatment with melatonin J Transl Med. 2019 Mar 12;17(1):77.
M-A Quera-Salva1, B Claustrat2[Melatonin: Physiological and pharmacological aspects related to sleep: The interest of a prolonged-release formulation (Circadin ®) in insomnia] Encephale. 2018 Dec;44(6):548-557. doi: 10.1016/j.encep.2018.06.005. Epub 2018 Aug 11.
S Huysmans, M De Hert, F Desplenter [Melatonin and sleep disorders: Overview of literature and testing in psychiatric practice] Tijdschr Psychiatr. 2019;61(12):854-861.
Ferri CP, Prince M, Brayne C, et al; Alzheimer’s Disease International. Global
prevalence of dementia: A Delphi consensus study.
Carpentieri, A., Marchionatti, A., Areco, V., Perez, A.,
Centeno, V., & Tolosa de Talamoni, N. (2014). Antioxidant and antiapoptotic
properties of melatonin restore intestinal calcium absorption altered by
menadione. Molecular and cellular biochemistry, 387(1-2), 197–205.
https://doi.org/10.1007/s11010-013-1885-2
Tasdemir, S., Parlakpinar, H., Vardi, N., Kaya, E., & Acet, A.
(2013). Effect of endogen-exogenous melatonin and erythropoietin on
dinitrobenzene sulfonic acid-induced colitis. Fundamental & clinical
pharmacology, 27(3), 299–307.
https://doi.org/10.1111/j.1472-8206.2011.01016.x
Parisotto, E. B., Vidal, V., García-Cerro, S., Lantigua, S.,
Wilhelm Filho, D., Sanchez-Barceló, E. J., Martínez-Cué, C., & Rueda, N. (2016).
Chronic Melatonin Administration Reduced Oxidative Damage and Cellular
Senescence in the Hippocampus of a Mouse Model of Down Syndrome. Neurochemical
research, 41(11), 2904–2913.
https://doi.org/10.1007/s11064-016-2008-8
Necefli, A., Tulumoğlu, B., Giriş, M., Barbaros, U., Gündüz,
M., Olgaç, V., Güloğlu, R., & Toker, G. (2006). The effect of melatonin on TNBS-induced
colitis. Digestive diseases and sciences, 51(9), 1538–1545.
https://doi.org/10.1007/s10620-005-9047-3
Trotta, R. J., Lemley, C. O., Vonnahme, K. A., & Swanson, K.
C. (2021). Effects of nutrient restriction and melatonin supplementation from
mid-to-late gestation on maternal and fetal small intestinal carbohydrase
activities in sheep. Domestic animal endocrinology, 74, 106555.
https://doi.org/10.1016/j.domaniend.2020.106555
Radwan P1, Skrzydlo-Radomanska B, Radwan-Kwiatek K, Burak-Czapiuk B, Strzemecka
J Is melatonin involved in the irritable bowel syndrome? J Physiol Pharmacol.
2009 Oct;60 Suppl 3:67-70.
Mozaffari S1, Rahimi R, Abdollahi M. Implications of melatonin therapy in
irritable bowel syndrome: a systematic review. Curr Pharm Des.
2010;16(33):3646-55.
Li, J., Li, R. X., Liu, G., Lv, C. F., Mi, Y. L., & Zhang, C.
Q. (2017). Effect of melatonin on renewal of chicken small intestinal mucosa.
Poultry science, 96(8), 2942–2949. https://doi.org/10.3382/ps/pex085
Desbonnet etal, 2012
Physiological and
behavioural responsivity to stress and anxiogenic stimuli in COMT-deficient mice
- PubMed (nih.gov)
Pertegal et al, 2016 Fetal
Val108/158Met catechol-O-methyltransferase (COMT) polymorphism and placental
COMT activity are associated with the development of preeclampsia - PubMed
(nih.gov)
Matos etal. 2016
Epistatic Interaction of
CYP1A1 and COMT Polymorphisms in Cervical Cancer - PubMed (nih.gov)
Ghareghani M, Sadeghi H, Zibara K, Danaei N, Azari H, Ghanbari A. Melatonin
Increases Oligodendrocyte Differentiation in Cultured Neural Stem Cells. Cell
Mol Neurobiol. 2017 Oct;37(7):1319-1324. doi: 10.1007/s10571-016-0450-4. Epub
2016 Dec 16. PMID: 27987059
Turgut M, Kaplan S. Effects of melatonin on peripheral nerve regeneration.
Recent Pat Endocr Metab Immune Drug Discov. 2011 May;5(2):100-8. doi:
10.2174/187221411799015336. PMID: 22074585.
Olivier P, Fontaine RH, Loron G, Van Steenwinckel J, Biran V, Massonneau V,
Kaindl A, Dalous J, Charriaut-Marlangue C, Aigrot MS, Pansiot J, Verney C,
Gressens P, Baud O. Melatonin promotes oligodendroglial maturation of injured
white matter in neonatal rats. PLoS One. 2009 Sep 22;4(9):e7128. doi:
10.1371/journal.pone.0007128. PMID: 19771167; PMCID: PMC2742165.
Russell-Jones, GJ, 2022
https://opastpublishers.com/open-access/functional-vitamin-b12-deficiency-in-sleep-disorders-in-children.pdfOkawa M, Mishima K, Nanami T, Shimizu T, Iijima S, Hishikawa Y, Takahashi K. Vitamin B12 treatment for sleep-wake rhythm disorders. Sleep. 1990 Feb;13(1):15-23. doi: 10.1093/sleep/13.1.15. PMID: 2305167.
Ohta T, Ando K, Iwata T, Ozaki N, Kayukawa Y, Terashima M, Okada T, Kasahara Y. Treatment of persistent sleep-wake schedule disorders in adolescents with methylcobalamin (vitamin B12). Sleep. 1991 Oct;14(5):414-8. PMID: 1759094.
Hysing M, Strand TA, Chandyo RK, Ulak M, Ranjitkar S, Schwinger C, Shrestha M, Kvestad I. The effect of vitamin B12-supplementation on actigraphy measured sleep pattern; a randomized control trial. Clin Nutr. 2022 Feb;41(2):307-312. doi: 10.1016/j.clnu.2021.11.040. Epub 2021 Dec 6. PMID: 34999324.
Takahashi K, Okawa M, Matsumoto M, Mishima K, Yamadera H, Sasaki M, Ishizuka Y, Yamada K, Higuchi T, Okamoto N, Furuta H, Nakagawa H, Ohta T, Kuroda K, Sugita Y, Inoue Y, Uchimura N, Nagayama H, Miike T, Kamei K. Double-blind test on the efficacy of methylcobalamin on sleep-wake rhythm disorders. Psychiatry Clin Neurosci. 1999 Apr;53(2):211-3. doi: 10.1046/j.1440-1819.1999.00534.x. PMID: 10459691.
Okawa M, Takahashi K, Egashira K, Furuta H, Higashitani Y, Higuchi T, Ichikawa H, Ichimaru Y, Inoue Y, Ishizuka Y, Ito N, Kamei K, Kaneko M, Kim Y, Kohsaka M, Komori T, Kotorii T, Matsumoto M, Mishima K, Mizuki Y, Morimoto K, Nagayama H, Ohta T, Okamoto N, Takahashi S, et al. Vitamin B12 treatment for delayed sleep phase syndrome: a multi-center double-blind study. Psychiatry Clin Neurosci. 1997 Oct;51(5):275-9. doi: 10.1111/j.1440-1819.1997.tb03198.x. PMID: 9413873.
Copyright © 2018 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited